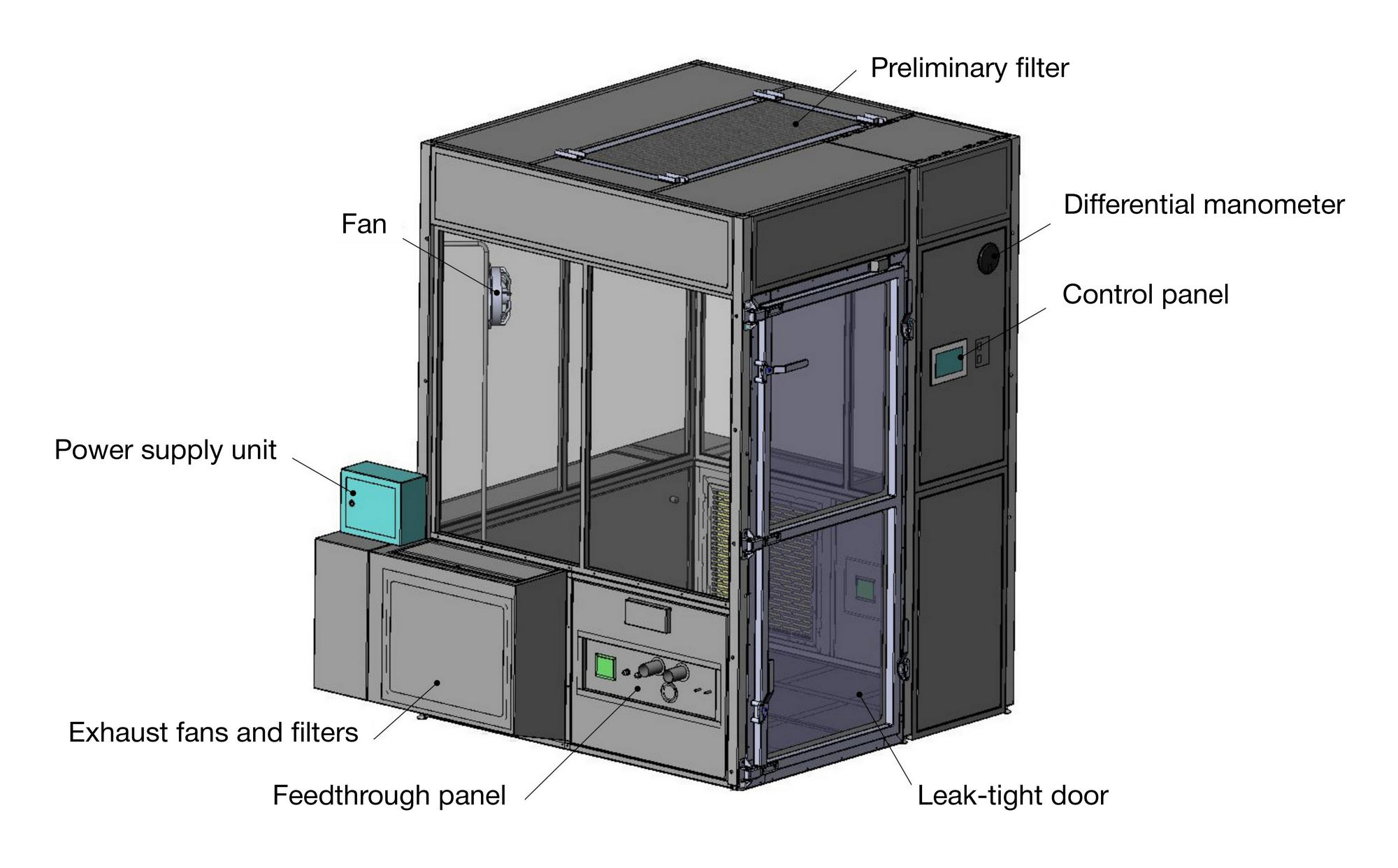
METHOD
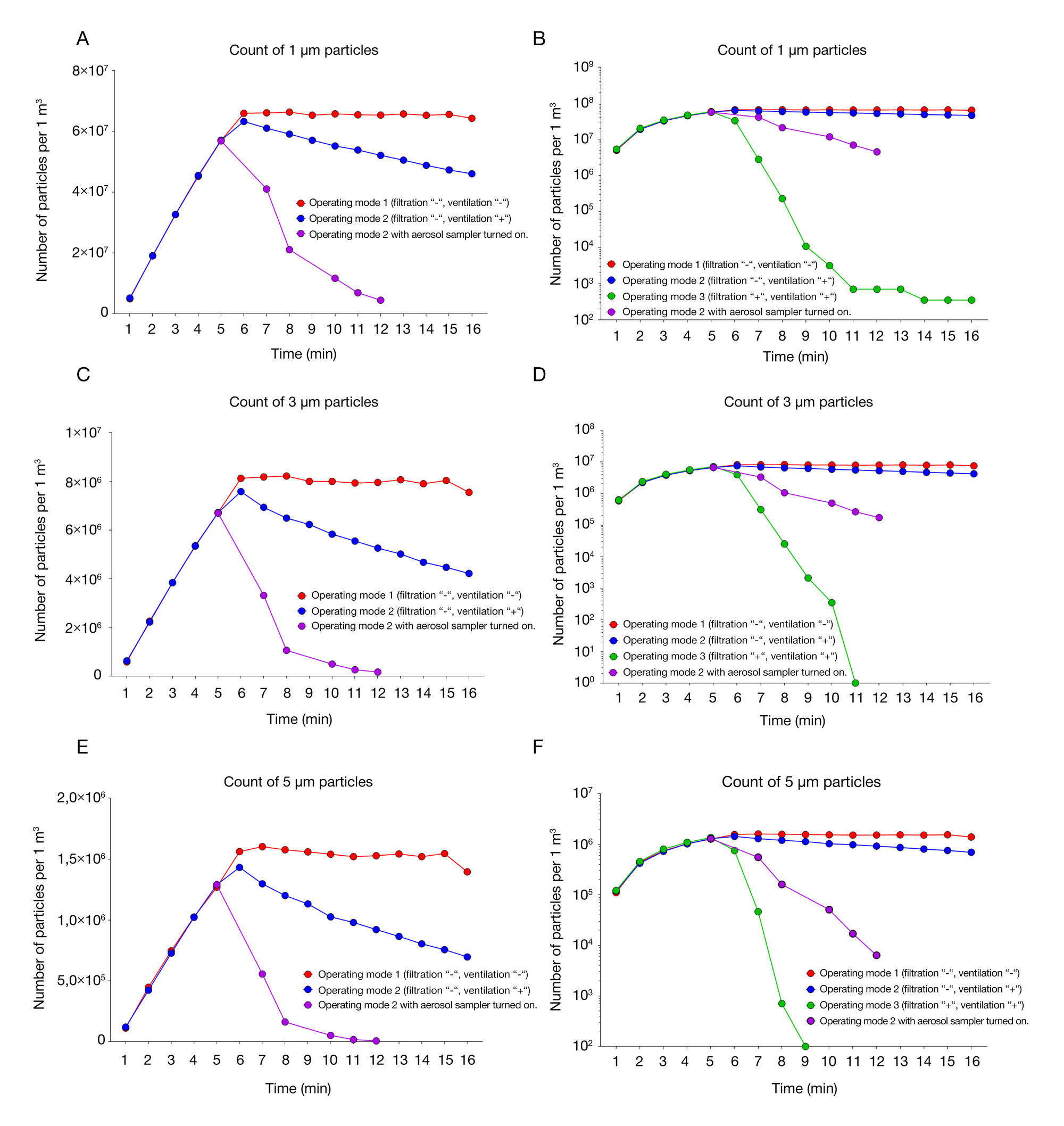
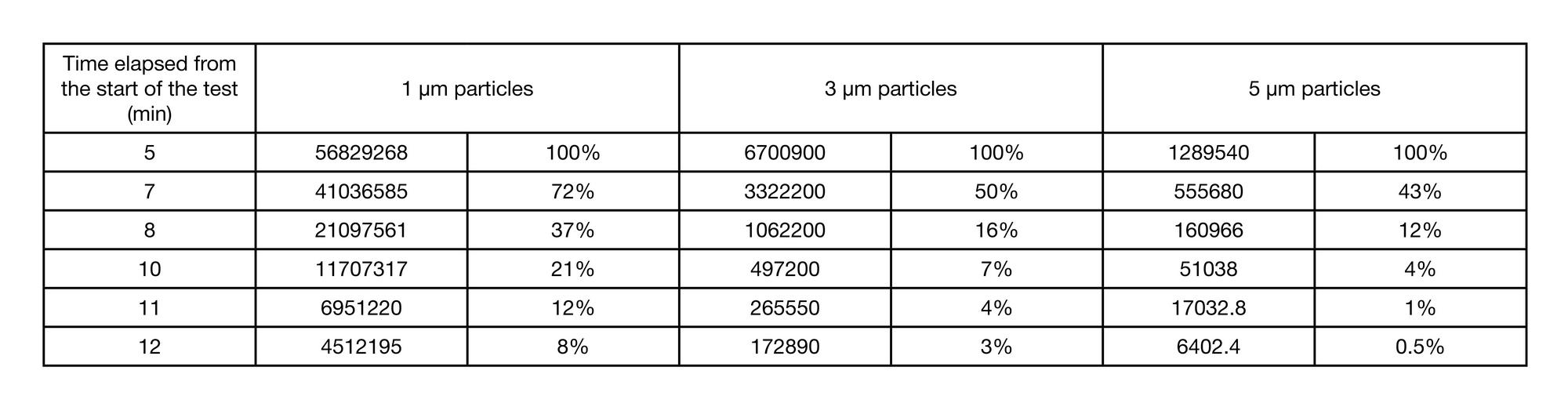
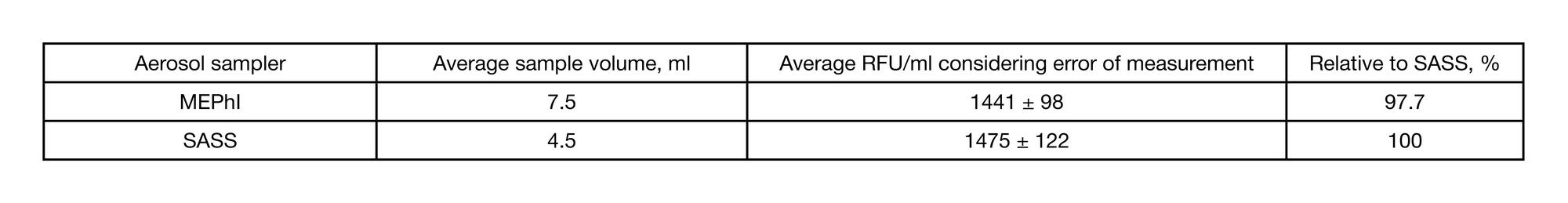
Performance of the original workstation for aerosol tests under controlled conditions
1 Translational Biomedicine Laboratory,
N. F. Gamaleya Federal Research Centre for Epidemiology and Microbiology, Moscow
2 ZAO Laminar Systems, Miass, Chelyabinsk oblast
3 Laboratory of Population Variability Mechanisms in Pathogenic Microorganisms,
N. F. Gamaleya Federal Research Centre for Epidemiology and Microbiology, Moscow
4 Department of Virology, Faculty of Biology, Lomonosov Moscow State University, Moscow
Correspondence should be addressed: Denis A. Kleymenov
Gamalei 18, Moscow, 123098; ur.relbmar@tel00001, gro.ayelamag@vonemyelk.a.sined
Funding: this work was supported by the Ministry of Health of the Russian Federation as part of the project The National System for Chemical and Biological Security of the Russian Federation (2015-2020) and by the Ministry of Education and Science as part of the project RFMEFI60117X0018.