OPINION
Modern tumor imaging models for rodents: potential and prospects in translational medicine
1 Shemyakin–Ovchinnikov Institute of Bioorganic Chemistry of the Russian Academy of Sciences, Moscow, Russia
2 Pirogov Russian National Research Medical University, Moscow, Russia
Rodent neoplastic process models are extensively used in pre-clinical practice to assess the dynamics of tumor development and test anti-cancer drugs, which ensures flexibility of choosing both basic and advanced personalized tumor development models for researchers. Various modern model tumor imaging methods are considered, including fluorescence and bioluminescence imaging, which enable comprehensive assessment, from qualitative evaluation to in vivo monitoring. We believe that the development of autonomous bioluminescent systems in mammalian cells will provide new possibilities for noninvasive imaging of animal physiological processes, including long-term monitoring of tumor progression.
Keywords: bioluminescence, bioimaging, biomedical research, autonomous bioluminescence, tumor models
Funding: the study was supported by the Russian Science Foundation grant, project No. 24-74-10105 (https://rscf.ru/project/24-74-10105/).
Author contribution: Fadeeva AA — literature review, manuscript writing; Myshkina NM — paper concept, literature review, manuscript writing, project management; Chepurnykh TV — manuscript writing and editing; Osipova ZM — data processing, manuscript editing.
Correspondence should be addressed: Nadezhda M. Myshkina
Miklukho-Maklaya, 16/10, Moscow, 117997, Russia; moc.liamg@aydan.anikram
Cancer represents one of the most diverse categories in terms of both tumor development and treatment. Despite this fact, it is possible to distinguish common traits and even model the disease course in other animal species (for example, in rodents) for many types of neoplasms. Given obvious differences in anatomy and genome structure between humans and rodents, such models are still optimal for pre-clinical trials [1]. The neoplastic process modeling is constantly evolving and has changed considerably over more than half a century since the first models were reported. As in other fields of biomedical science, the researchers prefer methods allowing them to obtain more precise information using a lower number of experimental objects and with fewer costs. Thus, the focus is shifting towards life-time noninvasive methods to assess model tumors using simple equipment. In particular, fluorescence and bioluminescence imaging methods enable long-term highsensitivity monitoring of tumor development and treatment in rodents. An example of the tumor suitable for such monitoring is the highly invasive fluorescent/bioluminescent patientderived orthotopic model of glioblastoma in mice created under the direction of Vladimir Baklaushev [2]. For the first time the researchers managed to noninvasively record tumor development in less than a week after inoculation and then trace its development by two orthogonal imaging methods (bioluminescence and fluorescence) at once.
Current review is aimed at considering modern rodent tumor models and imaging methods for those, as well as assuming which methods will develop most actively in the near future, providing researchers with the broadest opportunities.
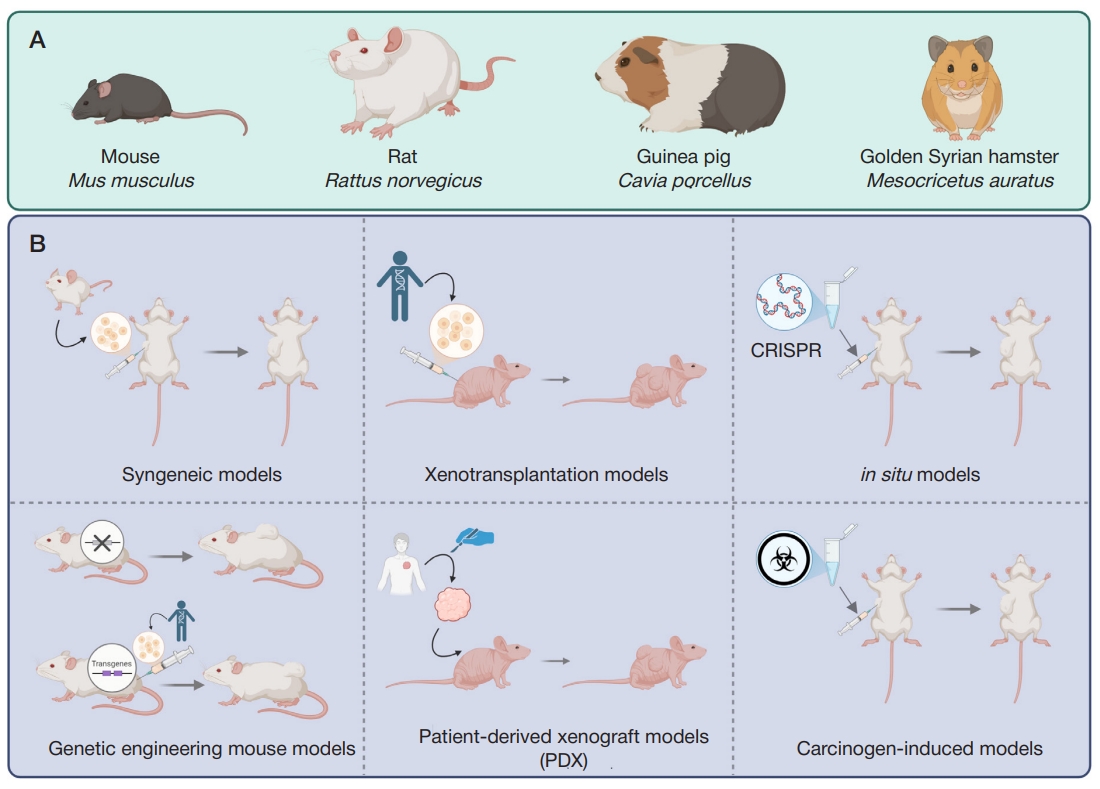
A wide selection of model animals and typical tumor models makes it possible to choose an optimal approach for each particular trial. The main model rodent species include mice (Mus musculus) [3], rats (Rattus norvegicus) [4], guinea pigs (Cavia porcellus), and Syrian golden hamsters (Mesocricetus auratus) [5] (figureА). However, the list is not limited to these species: marmots, voles, squirrels, degus, and other rodents are also used [6]. The methods to create model tumors include syngeneic models, xenotransplantation models, in situ tumor models, genetic engineering mouse models, patient-derived xenograft models (PDX), and the carcinogen-induced cancer model [4] (figureB).
Syngeneic models and xenograft models have been known since the 1960s. These are tumors developing from in vitro cultured transformed cells introduced into the model organism; depending on the model type, the organism species are either the same (syngeneic models), or different (xenotransplantation models) — in the latter case immunodeficient model animals are used. Despite the fact that such models fail to reliably reproduce tumor microenvironment, these are suitable for primary testing of anti-tumor drugs. Furthermore, syngeneic models of mouse tumors can be used to assess the efficacy of CAR-T therapy of solid tumors [7].
The patient-derived xenograft model is largely similar to the above models. However, a model immunodeficient animal is injected with tumor biopsy fragments obtained directly from the patient for experimental selection of personalized therapy. Such an approach allows one to identify response biomarkers and treatment targets for the tumor subgroups that can be distinguished based on the molecular profile [8].
In situ modeling that makes it possible to achieve tumor development in a certain organ through local gene editing largely overlaps with the carcinogen-induced cancer models that can also be local. Both approaches are used to develop the animal’s own tumor, which ensures better reproduction of, inter alia, blood supply. However, the use of the approaches for simulation of human carcinogenesis is limited. An example of the carcinogen-induced mouse model is a novel colitisassociated colorectal cancer mouse model obtained through intraperitoneal administration of azoxymethane to the CD4-dnTGFβRII mice [9].
Finally, all the variants of mice with the sustainably modified genotype are used in the genetic engineering mouse models: in some cases, these are more prone to spontaneous carcinogenesis in certain organs due to knockout of regulatory genes, in other cases these mimic human immune system (humanized mice) and are optimal for introduction of human tumor cells. Long production cycles and high cost can be considered the shortcomings of genetic engineering mouse models. An example of the genetic engineering mouse model is the mouse lineage overexpressing the luteinizing hormone receptor, which is prone to spontaneous endometrial cancer development [10].
Imaging of neoplastic processes in animal models has evolved from standard assessment of tumor location and size to advanced multimodal assessment of molecular, physiological, genetic, immunological, and biochemical events at microscopic and macroscopic levels performed noninvasively and sometimes in the real-time mode [11]. In contrast to necropsy, noninvasive imaging methods allow one to assess a tumor lesion in the body without having to sacrifice the experimental animal. Owing to noninvasive nature, the long-term monitoring becomes available yielding more accurate results and reducing the number of the animals needed [12]. Recently, such imaging techniques as magnetic resonance imaging (MRI), micro computed tomography (micro-CT), bioluminescence and fluorescence imaging, positron emission tomography (PET), etc. have become available for tumor imaging in small animals. Multimodal imaging allows one to trace structural and physiological alterations, metabolic processes, transplanted cells and target molecules, as well as to accomplish tumor imaging in distinct organs and the entire animal’s body. The use of nanoparticles is a powerful supplementary tool for the above methods due to high resolution, selectivity, and versatile nature [13, 14].
Among all imaging methods, MRI is one of the most informative due to high resolution and excellent contrast, which can be enhanced by adding exogenous paramagnetic contrast agents. This method enables detection of extrasmall tumors (up to 0.2 mm in diameter) in well-structured tissues and represents a “gold standard” for orthotopic brain tumors; it is also widely used for detection of metastases in other soft tissues, including the liver and lungs [15]. It has been shown that the weight of colorectal carcinoma calculated based on MRI data in vivo is rather accurately correlated to the carcinoma weight measured ex vivo, while the correlation with the bioluminescence signal intensity is rather weak, and the difference between the correlations is considerable. However, high cost and limitation of monitoring the neoplastic process at early stages after implantation are definitely the MRI shortcomings [16].
Compared to MRI, CT is considerably inferior in soft tissue and organ recognition quality. However, the main advantages of micro-CT are its high resolving power (<50 µm) and rapid visualization of the lung and bone tissue allowing one to detect cancerous neoplasms. Since bones represent a common metastatic focus for the major types of malignant neoplasms (including breast and prostate carcinomas), some studies report the use of high-resolution (10 µm) micro-CT for detection of metastases of breast carcinomas of different etiology to the bone tissue [17]. Moreover, micro-CT is particularly well suited for acquisition of high-quality anatomic information about the lungs [18]. Thus, the use of 3D analysis allows one to obtain accurate data on the tumor number, size, and progression. It is superior to conventional histology or lung resection. Extra administration of contrast agents is needed to record soft tissue tumors, which complicates the procedure [19].
Widely used methods also include nuclear diagnostic procedures, during which images are acquired by introducing the short-lived radioisotopes and recording their decay using SPECT or PET scanner, which eventually shows spatial and temporal distribution of radioactive substances and drugs specific for certain targets. PET with 18F-fluorodeoxyglucose (18FDG) is the most common imaging method used in both pre-clinical and clinical trials [20]. This method is characterized by high specificity and sensitivity, as well as by positive prognostic value when used to detect tumors. At the same time, it is necessary to somehow prepare model animals for the 18FDG-PET scan; in particular, strict dietary restrictions are necessary to ensure that basal glucose metabolism levels do not mask the test results [21].
Fluorescence imaging is used for visualization of biological processes throughout the body involving the use of the genetically encoded fluorescent proteins or fluorescent dyes [22]. Bioluminescence imaging also represents a noninvasive imaging method based on biochemical reaction of the substrate (luciferin) oxidation by atmospheric oxygen under exposure to specific enzyme (luciferase) with light emission. In contrast to fluorescence, bioluminescence does not require any external light source and, therefore, does not have the related side effects, such as autofluorescence and photobleaching [23]. Obvious advantages of bioluminescence imaging include relative simplicity of use, image acquisition speed, lower cost compared to MRI, and high sensitivity, which makes the method popular in pre-clinical trials. Potential shortcomings of the method include the need for genetic modification of the cells and the need for exogenous substrate supplementation. It should be noted that both bioluminescence and fluorescence imaging methods show high efficacy when used to detect small nonpalpable tumors, but both methods suffer from the signal decrease due to tissue absorption and scattering, which limits the depth at which tumor visualization is possible and hampers acquisition of quantitative data from deeper tissues [24].
It is possible to overcome fundamental flaws in bioluminescence imaging of model tumors in vivo through a shift towards autonomous bioluminescent systems. Such systems do not require exogenous substrate supplementation, which both reduces the cost of their use and simplifies measurement. The fundamental possibility of creating a fully autonomous bioluminescent mouse has been confirmed [25], and tumors of such mice are suitable for production of at least syngeneic models. There is also a possibility of gene modification of the tumor cells only for autonomous bioluminescence. In this case, viruses specifically targeting cancer cells may be used to deliver genes of autonomous bioluminescent systems: depending on cancer type these may include adenoviruses, poxviruses, herpes simplex virus 1 (HSV-1), coxsackieviruses, poliovirus, measles virus, Newcastle disease virus (NDV), reoviruses, etc. [26]. Delivery of appropriate mRNA using lipid nanoparticles may become an alternative [14]. It is possible to improve the method sensitivity via treatment of rodent skin with specific dyes showing high absorption in violet and blue spectral regions, which reversibly make the skin transparent in visible light while alive [27, 28].
Today, the key shortcoming is low brightness of autonomous bioluminescent systems, however, research teams in different countries are working to improve these, specifically due to the use of natural orthologs of essential enzymes or their modifications obtained by site-directed mutagenesis [29]. We believe that in the future autonomous bioluminescent models of rodent tumors will be introduced widely into pre-clinical practice along with conventional models.
CONCLUSION
Modern methods to create rodent tumor models reported in this review combined with innovative imaging approaches have expanded the possibilities of pre-clinical trials. In the near future, autonomous bioluminescent models that use the genetically encoded luciferases and luciferin biosynthesis enzymes for noninvasive tumor monitoring have a significant development potential. Such systems enable long-term monitoring with high spatiotemporal resolution without the need for exogenous substrate supplementation, which is especially important for investigation of metastases and monitoring of tumor alterations when treating with therapeutic agents. The approaches based on autonomous bioluminescence can become a valuable translational oncology tool, contributing to a shift towards more personalized pre-clinical trials.
- Hollingshead MG. Antitumor efficacy testing in rodents. J Natl Cancer Inst. 2008; 100: 1500–10. DOI: 10.1093/jnci/djn351.
- Yuzhakova D, Kiseleva E, Shirmanova M, Shcheslavskiy V, Sachkova D, Snopova L, et al. Highly invasive fluorescent/bioluminescent patient-derived orthotopic model of glioblastoma in mice. Front Oncol. 2022; 12: 897839. DOI: 10.3389/fonc.2022.897839.
- Utz B, Turpin R, Lampe J, Pouwels J, Klefström J. Assessment of the WAP-Myc mouse mammary tumor model for spontaneous metastasis. Sci Rep. 2020; 10: 18733. DOI: 10.1038/s41598-020-75411-z.
- Guo H, Xu X, Zhang J, Du Y, Yang X, He Z, et al. The pivotal role of preclinical animal models in anti-cancer drug discovery and personalized cancer therapy strategies. Pharmaceuticals (Basel). 2024; 17: 1048. DOI: 10.3390/ph17081048.
- Wang Z, Cormier RT. Golden Syrian hamster models for cancer research. Cells. 2022; 11: 2395. DOI: 10.3390/cells11152395.
- Jackson RK. Unusual laboratory rodent species: Research uses, care, and associated biohazards. ILAR J. 1997; 38: 13–21. DOI: 10.1093/ilar.38.1.13.
- Ahmed EN, Cutmore LC, Marshall JF. Syngeneic mouse models for pre-clinical evaluation of CAR T cells. Cancers (Basel). 2024; 16: 3186. DOI: 10.3390/cancers16183186.
- Zanella ER, Grassi E, Trusolino L. Towards precision oncology with patient-derived xenografts. Nat Rev Clin Oncol. 2022; 19: 719–32. DOI: 10.1038/s41571-022-00682-6.
- Uragami T, Ando Y, Aoi M, Fukui T, Matsumoto Y, Horitani S, et al. Establishment of a novel colitis-associated cancer mouse model showing flat invasive neoplasia. Dig Dis Sci. 2023; 68: 1885–93. DOI: 10.1007/s10620-022-07774-4.
- Lottini T, Iorio J, Lastraioli E, Carraresi L, Duranti C, Sala C, et al. Transgenic mice overexpressing the LH receptor in the female reproductive system spontaneously develop endometrial tumour masses. Sci Rep. 2021; 11: 8847. DOI: 10.1038/s41598-021-87492-5.
- Serkova NJ, Glunde K, Haney CR, Farhoud M, De Lille A, Redente EF, et al. Preclinical applications of multi-platform imaging in animal models of cancer. Cancer Res. 2021; 81: 1189–200. DOI: 10.1158/0008-5472.CAN-20-0373.
- Bausart M, Bozzato E, Joudiou N, Koutsoumpou X, Manshian B, Préat V, et al. Mismatch between bioluminescence imaging (BLI) and MRI when evaluating glioblastoma growth: Lessons from a study where BLI suggested “regression” while MRI showed “progression”. Cancers (Basel). 2023; 15: 1919. DOI: 10.3390/cancers15061919.
- Yin C, Hu P, Qin L, Wang Z, Zhao H. The current status and future directions on nanoparticles for tumor molecular imaging. Int J Nanomedicine. 2024; 19: 9549–9574. DOI: 10.2147/IJN.S484206.
- Liu B, Zhou H, Tan L, Siu KTH, Guan X-Y. Exploring treatment options in cancer: Tumor treatment strategies. Signal Transduct Target Ther. 2024; 9: 175. DOI: 10.1038/s41392-024-01856-7.
- Green AL, DeSisto J, Flannery P, Lemma R, Knox A, Lemieux M, et al. BPTF regulates growth of adult and pediatric high-grade glioma through the MYC pathway. Oncogene. 2020; 39: 2305– 27. DOI: 10.1038/s41388-019-1125-7.
- Ravoori MK, Margalit O, Singh S, Kim S-H, Wei W, Menter DG, et al. Magnetic resonance imaging and bioluminescence imaging for evaluating tumor burden in orthotopic colon cancer. Sci Rep. 2019; 9: 6100. DOI: 10.1038/s41598-019-42230-w.
- Previdi S, Abbadessa G, Dalò F, France DS, Broggini M. Breast cancer-derived bone metastasis can be effectively reduced through specific c-MET inhibitor tivantinib (ARQ 197) and shRNA c-MET knockdown. Mol Cancer Ther. 2012; 11: 214–23. DOI: 10.1158/1535-7163.MCT-11-0277.
- Pennati F, Leo L, Ferrini E, Sverzellati N, Bernardi D, Stellari FF, et al. Micro-CT-derived ventilation biomarkers for the longitudinal assessment of pathology and response to therapy in a mouse model of lung fibrosis. Sci Rep. 2023; 13: 4462. DOI: 10.1038/s41598-023-30402-8.
- Tan MJ, Fernandes N, Williams KC, Ford NL. In vivo microcomputed tomography imaging in liver tumor study of mice using Fenestra VC and Fenestra HDVC. Sci Rep. 2022; 12: 22399. DOI: 10.1038/s41598-022-26886-5.
- Hesketh RL, Wang J, Wright AJ, Lewis DY, Denton AE, Grenfell R, et al. Magnetic resonance imaging is more sensitive than PET for detecting treatment-induced cell death-dependent changes in glycolysis. Cancer Res. 2019; 79: 3557–69. DOI: 10.1158/0008-5472.CAN-19-0182.
- Toner YC, Prévot G, van Leent MMT, Munitz J, Oosterwijk R, Verschuur AVD, et al. Macrophage PET imaging in mouse models of cardiovascular disease and cancer with an apolipoproteininspired radiotracer. Npj Imaging. 2024; 2: 12. DOI: 10.1038/s44303-024-00009-3.
- Ito R, Kamiya M, Urano Y. Molecular probes for fluorescence image-guided cancer surgery. Curr Opin Chem Biol. 2022; 67: 102112. DOI: 10.1016/j.cbpa.2021.102112.
- Townsend KM, Prescher JA. Recent advances in bioluminescent probes for neurobiology. Neurophotonics. 2024; 11: 024204. DOI: 10.1117/1.NPh.11.2.024204.
- Gleneadie HJ, Dimond A, Fisher AG. Harnessing bioluminescence for drug discovery and epigenetic research. Front Drug Discov (Lausanne). 2023; 3: 1249507. DOI: 10.3389/fddsv.2023.1249507.
- Kiszka KA, Dullin C, Steffens H, Koenen T, Rothermel E, Alves F, et al. Autonomous bioluminescence emission from transgenic mice. bioRxiv. 2024. DOI: 10.1101/2024.06.13.598801.
- Kaufman HL, Kohlhapp FJ, Zloza A. Oncolytic viruses: a new class of immunotherapy drugs. Nat Rev Drug Discov. 2016; 15: 660. DOI: 10.1038/nrd.2016.178.
- Ou Z, Duh Y-S, Rommelfanger NJ, Keck CHC, Jiang S, Brinson K Jr, et al. Achieving optical transparency in live animals with absorbing molecules. Science. 2024; 385: eadm6869. DOI: 10.1126/science.adm6869.
- Keck CHC, Schmidt EL, Roth RH, Floyd BM, Tsai AP, Garcia HB, et al. Color-neutral and reversible tissue transparency enables longitudinal deep-tissue imaging in live mice. bioRxivorg. 2025. p. 2025.02.20.639185. DOI: 10.1101/2025.02.20.639185.
- Shakhova ES, Karataeva TA, Markina NM, Mitiouchkina T, Palkina KA, Perfilov MM, et al. An improved pathway for autonomous bioluminescence imaging in eukaryotes. Nat Methods. 2024; 21: 406–10. DOI: 10.1038/s41592-023-02152-y.