ORIGINAL RESEARCH
Intranasal lipopolysaccharide administration to Sprague-Dawley rats as a biomodel of acute respiratory distress syndrome
1 Patrice Lumumba Peoples' Friendship University of Russia, Moscow, Russia
2 Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Moscow, Russia
3 Petrovsky National Research Centre of Surgery, Moscow, Russia
High fatality rate and the lack of pathophysiological therapy are typical for acute respiratory distress syndrome (ARDS). Intratracheal lipopolysaccharide (LPS) administration is used to model ARDS in animals. The method has the limitation of requiring the use of equipment to perform intubation and control the animal’s state. The study aimed to assess the possibility of using intranasal LPS administration instead of intratracheal and determine the LPS optimal dose. A total of 150 mL of the E. coli O111:B4 LPS (7.5 mg/kg or 15 mg/kg) or 0.9% NaCl was administered to 21 Sprague-Dawley rats. After 48 h blood was collected from the tail vein to determine the white blood cell count and TNFa concentration. The lungs were retrieved to assess dry weight (wet/dry ratio) and to determine the expression of the genes encoding pro- and anti-inflammatory cytokines using real-time PCR. The relative counts of CD68-, CD86-, and MHC II-positive cells in the lung tissue were also evaluated using flow cytometry. The w/d ratio was higher when the dose of 15 mg/kg of body weight was used (p = 0.0228, ordinary one-way Anova). Вlood lymphocyte counts were decreased (p = 0.0019, ordinary one-way Anova), and neutrophil counts were increased (p = 0.0021, ordinary one-way Anova) upon administration of both doses. The counts of CD86- (p = 0.0014, ordinary one-way Anova) and MHC II-positive cells (p = 0.0050, ordinary one-way Anova) increased after LPS administration. The IL10 gene expression was significantly increased upon administration of the dose of 15 mg/kg (p = 0.0024, ordinary oneway Anova), while the IL4 expression (p = 0.0194, ordinary one-way Anova) was decreased upon administration of the dose of 7.5 mg/kg. Thus, intranasal LPS administration can be used to model ARDS in the Sprague-Dawley rats. Administration of the high dose leads to the rapid development of inflammation in the lung.
Keywords: LPS, animal model, lipopolysaccharide, Sprague-Dawley rats, cytokine, ARDS, expression level
Funding: the study was supported by the Russian Science Foundation (grant No. 24-25-00203).
Acknowledgements: the authors would like to thank D.A. Areshidze, C. Sci. Biol., Head of the Cellular Pathology Laboratory of the Avtsyn Institute of Human Morphology for performing the complete blood count test in animals.
Author contribution: Kiseleva VV — experimental design and procedure, analysis of the results, manuscript writing; Vishnyakova PA — advice on the experimental procedure, material resources, editing; Kosyreva AM — advice on the experimental procedure, editing; Kananykhina EYu, Emelianov II — animal handling; Elchaninov AV — advice on the experimental procedure, material resources, editing; Fatkhudinov TH — material resources for the study.
Compliance with ethical standards: the study was approved by the Ethics Committee of the Avtsyn Institute of Human Morphology (protocol No. 21 dated 29 March 2019). Animals were handled in accordance to the ARRIVE guidelines and the Directive ЕС 2010/63/EU on the protection of animals used for scientific purposes.
Correspondence should be addressed: Victoria V. Kiseleva
Oparina, 4, Moscow, 117997, Russia; moc.liamg@1991.avosonruk.airotciv
Acute lung injury and acute respiratory distress syndrome (ARDS), representing its most severe form, are complex, multifactorial disorders. Today, there is no pathophysiological therapy for ARDS, and treatment remains exclusively symptomatic, which makes studies of this disorder relevant [1]. The use of animal models is essential for both assessment of pathophysiological processes associated with the disease development and estimation of potential drug efficacy.
The effectiveness of human ARDS reproduction in an animal model is assessed based on the presence of major morphological alterations that are typical for the disorder: presence of intra-alveolar edema; elevated neutrophil counts in the interalveolar septa; hyaline membrane formation leading to the interalveolar septa thickening; formation of microthrombi [2]. The extent of these alterations is usually assessed within 24 h; longer times after the LPS administration remain understudied.
Various animal species, from rodents to anthropoid apes, are used to model ARDS [3]. ARDS is most often simulated in mice and rats due to maximum affordability and ease of handling. Several approaches to ARDS modeling are distinguished: direct and indirect lung injury (intranasal/intratracheal or intravenous administration of the bacterial wall lipopolysaccharide (LPS) of Gram-negative bacteria), as well as combined models.
LPS consists of three parts determining its immunogenicity. The А lipid “anchors” the LPS molecule in the cell wall of Gram-negative bacteria and binds the O-chain on the bacterial surface via the core polysaccharide. The O-chain is unique for each bacterium; it provides the basis for serotyping historically used for identification of Gram-negative bacteria. It is believed that bacteria that form smooth colonies have a less pyrogenic O-chain consisting of the repeating disaccharides, and LPS from the bacterium having no O-chain is the most immunogenic.
The benefit of using LPS to model ARDS is represented by its relative affordability and the possibility of standardizing the experiments. However, it is worth remembering that LPS formulations often contain contaminants, such as bacterial lipoproteins, which can affect the LPS biological effects.
After entering the body LPS is recognized by the Toll-like receptor 4 (TLR4) on the surface of monocytes, macrophages, and dendritic cells. The lipopolysaccharide-binding protein (LBP) and CD14, playing the role of co-receptors in this interaction, contribute to activation of the MyD88 and TRIFdependent signaling pathways [4]. These cascade reactions lead to activation of the transcription factors NF-κB/MAPK (mitogen-activated protein kinases) and IRF3, respectively. As a result, production of pro-inflammatory cytokines TNFα, IL6, IL1β and type I interferons is stimulated, which mediates the development of inflammation [4].
ARDS modeling involving the use of intratracheal LPS administration is currently used more often than intranasal administration, which results from the more targeted effect on the lower respiratory tract. However, such an approach requires the use of specific equipment for LPS delivery in the lower respiratory tract through the nose or through drug administration via the tracheal incision, which increases the animal's recovery time and bears the risk of the surgical wound infection. This may increase both the research time frame and the financial resources required to implement the research. Furthermore, there are data suggesting comparable effects of both administration methods. Comparison of intranasal and intratracheal administration of the O55:B5 Escherichia coli LPS was performed in 12 female C57Bl/6 J mice by the researchers from Canada guided by Fatemeh Khadangi. One LPS dose and withdrawal of animals from the experiment after 24 h were used. The authors showed that the administration route did not affect the inflammation severity; however, lower variability of the studied parameters (cell counts and total protein levels in bronchoalveolar lavage, dry lung weight, inflammation severity based on the lung histological slides, and mechanical ventilation indicators) within the group when using intranasal administration [5].
Other authors have shown that intratracheal administration of the E. coli O111:B4 LPS results in bronchopneumonia, increased counts of bone marrow-derived macrophages and macrophages with inflammatory phenotype in bronchoalveolar lavage. The development of local inflammation in the lung was reflected in high expression of pro-inflammatory cytokines and low expression of anti-inflammatory cytokines. High serum C-reactive protein levels were reported at the systemic level [6].
The study aimed to assess the possibility of using intranasal LPS administration to Sprague-Dawley (SD) rats for ARDS simulation and compare alterations in the rat lung after a single administration of LPS in a dose of 7.5 mg/kg or 15 mg/kg.
METHODS
Intranasal LPS administration
Male Sprague-Dawley rats weighting 250–280 g were obtained from the Stolbovaya breeding nursery (Moscow Region, Russia). The animals were kept with natural light, at a temperature of 20–22 °С and relative air humidity of 60–70%. The animals had ad libitum access to the drinking water and pelleted feed (Laboratorsnab LLC, Russia).
The study involved 21 animals. A total of 150 µL of the E. coli O111:B4 LPS (Sigma, USA), 7.5 mg/kg or 15 mg/kg, was administered intranasally to experimental animals under heavy injection anesthesia (Zoletil, Virbac, France). The control rats were administered 150 µL of saline (NaCl 0.9%).
Lungs of 10 rats were retrieved 48 h after administration of the 0.9% NaCl (three animals), LPS in a dose of 7.5 mg/kg of body weight (four animals), and LPS in a dose of 15 mg/kg of body weight (three animals) to determine dry weight.
In nine rats (the same groups, three animals per group), the right lung was retrieved 48 h after administration of LPS or 0.9% NaCl. A small part of those was placed in the RNA fixative (Evrogen, Russia) for further RNA extraction, performing RT and PCR aimed at estimating the local inflammation severity. The remaining lung was homogenized, fixed and stained in order to determine the CD68, CD86, and MHC II macrophage markers by flow cytometry. A total of 2–3 mL of blood was collected from the tail vein of the same animals for serum extraction and enzymelinked immunoassay aimed at assessing the TNFa concentration (Cloude-Clone, #SEA133Ra) and complete blood counts.
Finally, lungs of another three intact animals were used to determine macrophage markers by flow cytometry.
Wet/dry ratio estimation
To assess the edema severity, lungs of 10 animals were retrieved and weighted 48 h after administration of LPS or saline. Re-weighting was performed after 7 days of drying lungs at a temperature of 60 °C. The wet/dry ratio was calculated by dividing the lung weight 48 hours after LPS injection by the weight after drying [8].
RNA extraction, reverse transcription, and PCR
Total RNA was extracted from the samples of the right lung placed in the IntactRNA (Evrogen, Russia) using the Magzol reagent (Magen, China) in accordance with the manufacturer’s instructions in order to determine the expression of the genes encoding pro- (TNFα, IL18, IL13, IL1β) and anti-inflammatory (IL10, IL4) cytokines, genes of the enzymes involved in arginine metabolism (NOS2 and Arg1), and the gene of the NF-kb transcription factor. The first cDNA chain for determination of gene expression was obtained from 1 µg of total RNA using the MMLV RT kit (Evrogen, Russia) in accordance with the manufacturer’s instructions.
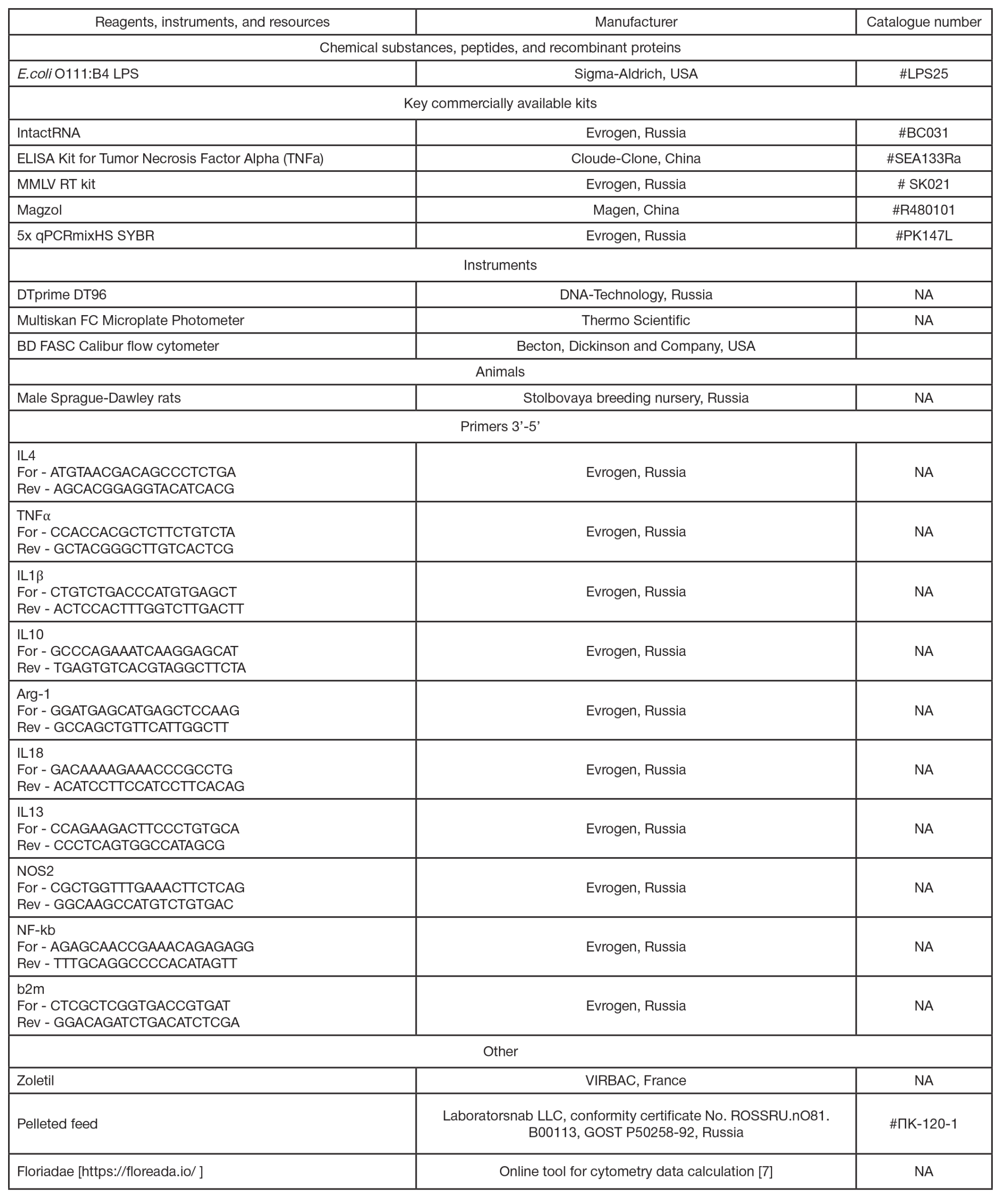
Real-time PCR was conducted in the volume of 25 µL containing 400 ng of the first cDNA chain using 400 nМ of forward and reverse primers (sequences are provided in tab. 1) and 5х qPCRmixHS SYBR (Evrogen, Russia). All the reactions were carried out in triplicate in the DTprime DT96 cycler (DNATechnology, Russia) under the following conditions: initial denaturation — 95 °C 5 min; 40 amplification cycles including denaturation — 95 °C 15 s, annealing — 60 °C 12 s, and elongation — 72 °C 15 s. Specificity of the product yielded was tested by the PCR product melting curve analysis using the Real Time PCR software tool (DNA-Technology, Russia). Threshold cycles (Ct) for all the studied genes were determined based on the fluorescent signal accumulation curve. Relative gene expression was calculated by the 2^(-ΔΔCt) method. The housekeeping beta-2 microglobulin gene was used to normalize the expression of each gene.
Flow cytometry
Levels of the pan-macrophagal marker CD68, CD86 typical for pro-inflammatory macrophages, and MHC II typical for antiinflammatory macrophages were assessed by flow cytometry. For that lungs of 12 animals (intact animals, animals post administration of the 0.9% NaCl and LPS in a dose of 7.5 mg/kg or 15 mg/kg; three animals per group) were mechanically homogenized, then passed through a cell strainer with the pore diameter of 100 µm to eliminate the remaining large fragments and fixed with the 2% paraformaldehyde. A total of 106 cells were used for permeabilization with the Inside Perm reagent (#130-090-477, Miltenyi, Germany). CD68 was stained with
FITC (#130-133-301, Miltenyi, Germany) or CD68 PE-Vio 770 (#130-134-152 Miltenyi, Germany), CD86 with Vio Bright FITC (#130-109-180 Miltenyi, Germany), and MHC II with PE (#205308, Biolegent). At least 50,000 events were acquired using a BD FACS Calibur flow cytometer (USA). The relative number of CD68, CD86 и MHC II-positive cells was assessed using the Floriadae online tool [7].
Enzyme-linked immunoasay
To determine serum TNFα concentration, 2 mL of blood were collected from the tail vein of the SD rat under heavy anesthesia into test tubes with clotting activator. Centrifugation was performed at 3200 rpm for 20 min. Then serum was collected in the new tubes and stored at –20 °С. Assay was performed using the ELISA Kit for Tumor Necrosis Factor Alpha (TNFa) (Cloude-Clone, China, #SEA133Ra) in accordance with the manufacturer’s instructions. Optical density was determined at the wavelength of 450 nm using the Multiskan FC Microplate Photometer (Thermo Scientific, USA).
Statistical analysis
Statistical analysis was performed using the Prism 8.0 software. The Mann–Whitney U test was used when comparing two groups; ordinary one-way ANOVA was used when comparing a larger number of groups. The differences were considered significant at p < 0.05. Box plots including the median and the upper/lower extremes were used for graphic representation of the data.
RESULTS
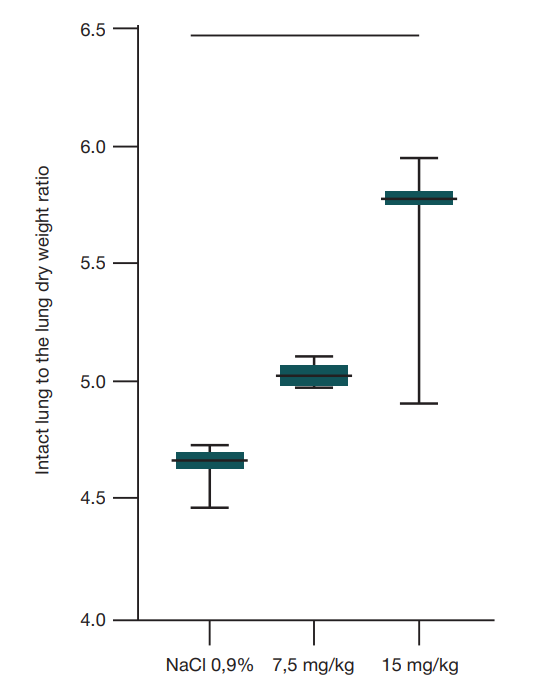
The development of acute lung injury was assessed primarily based on the wet-to-dry ratio (W/D), which is an objective indicator of lung tissue water content and reflects the development of the exudative phase of acute respiratory distress syndrome (ARDS). The entire lung was used to determine the W/D ratio. A significantly high W/D relative to the control group was determined after administration of the dose of 15 mg/kg of body weight (p = 0.0228, ordinary one-way Anova) (fig. 1).
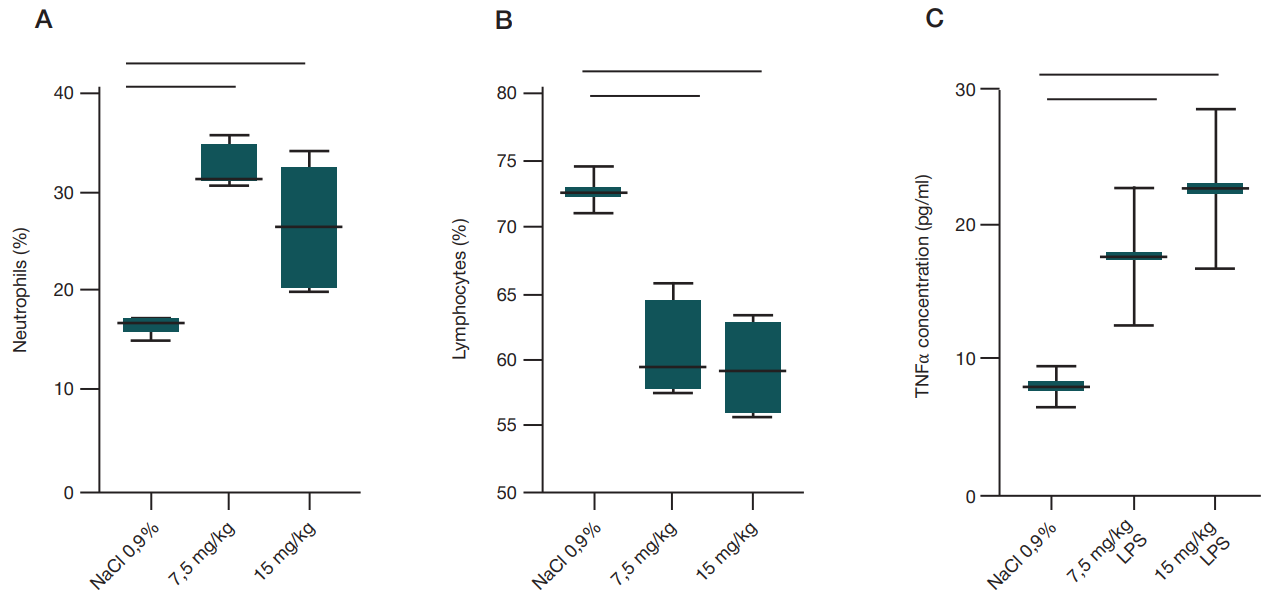
At the systemic level intranasal administration of both LPS doses resulted in the significantly low relative lymphocyte counts (p = 0.0019, ordinary one-way Anova) and significantly high neutrophil counts (p = 0.0021, ordinary one-way Anova).
After LPS administration the experimental rats showed a more than 4-fold increase in serum concentrations of the TNFα pro-inflammatory cytokine compared to the control group, however, the differences were non-significant (fig. 2).
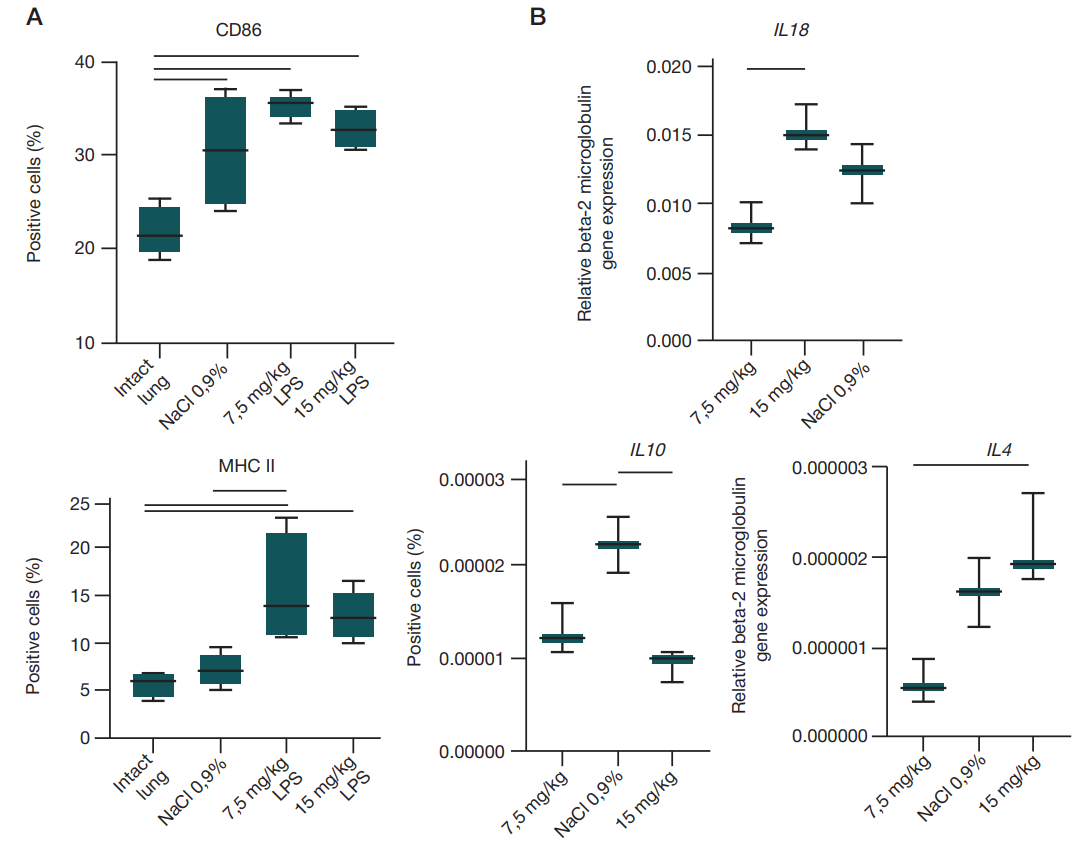
It is well known that macrophages determine the ARDS outcome. That is why the relative counts of cells carrying the CD68 pan-macrophagal marker and the markers reported for both pro-inflammatory and anti-inflammatory macrophages (CD86 and MHC II, respectively) were assessed in the rat lung homogenates during the next stage [9–11]. Experimental animals showed significantly high levels of CD86-positive cells in the lung relative to intact animals after administration of both LPS doses (p = 0.0014, ordinary one-way Anova); when the LPS dose of 7.5 mg/kg of body weight was used, the significantly high levels of MHC II-positive cells were also observed (p = 0.0050, ordinary one-way Anova) (fig. 3).
The development of local inflammation was assessed through determining the expression of genes of the TNFα, IL1β, IL13, and IL18 pro-inflammatory cytokines, gene of the NF-kb transcription factor, genes of IL10 and IL4 anti-inflammatory cytokines, as well as genes of the enzymes involved in arginine metabolism, NOS2 and Arg1 (fig. 3).
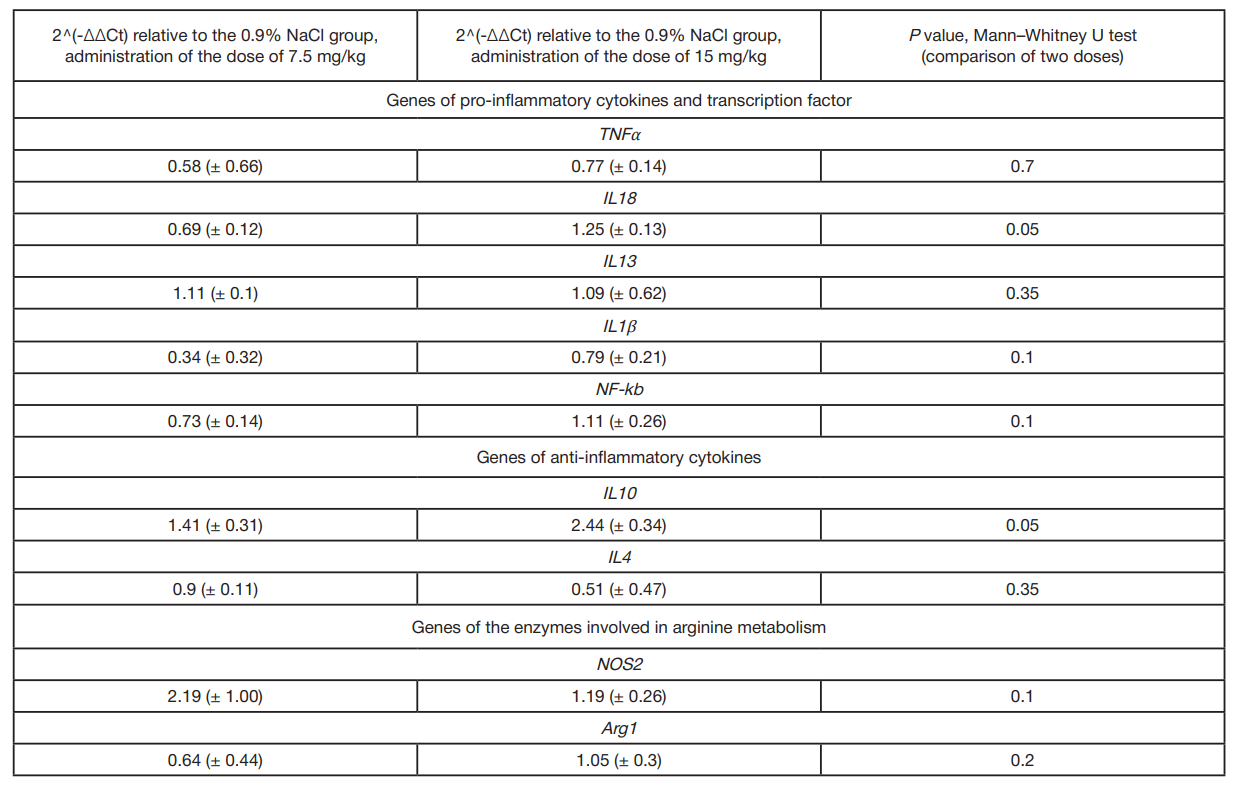
Expression of the IL10 gene was significantly increased in the experimental animals administered LPS in a dose of 15 mg/kg of body weight (ordinary one-way Anova, p = 0.0024), while the IL4 expression was decreased in those administered the dose of 7.5 mg/kg of body weight (ordinary one-way Anova, p = 0.0194) compared to the animals administered saline. As for genes IL18 and IL10, there were significant differences between the animals administered different LPS doses (ordinary one-way Anova, р = 0.009 and 0.0024, respectively).
Comparison of the extent of the expression change (2^(-ΔΔCt)) after administration of two LPS doses relative to the group administered saline using the Mann–Whitney U test showed a significant increase in expression of the IL18 pro-inflammatory cytokine and IL10 anti-inflammatory cytokine genes after administration of the dose of 15 mg/kg of body weight (tab. 2).
DISCUSSION
Modeling ARDS to study its pathogenesis and search for potential therapy is a non-trivial task, which is largely due to the pathogenetic features’ complex nature.
It is common to distinguish three disease phases, regardless of the cause: exudative, proliferative, and fibrotic. The ARDS exudative phase lasts 1–7 days. During this period the damaging factors lead to disruption of the blood-air barrier and the development of intra-alveolar edema [1]. In the majority of papers, neutrophil infiltration of the inter-alveolar septa and the development of intra-alveolar edema are assessed in the lung within 24 h. ARDS model validation within 48 h is a feature of this study. The significantly high ratio of the lung weight after retrieval of the lung from the animal’s body to its dry weight, when administered the E. coli O111:B4 LPS dose of 15 mg/kg of body weight, suggests high liquid content in the lung tissue, which indicates the exudative phase of the disease.
At the systemic level the signs of inflammatory response development were reported, which was indicated by the significantly low relative lymphocyte counts and significantly high neutrophil counts. Lymphocytes are cells of the adaptive immune system that help the body to withstand invasion of pathogenic microorganisms, including via attraction of neutrophils to the foci of infection during acute inflammation. The cascade of reactions resulting in bone marrow stimulation and the release of a large number of neutrophils into the circulatory system is triggered under the LPS exposure [12]. Low lymphocyte counts can result from redistribution of cells to the inflammatory focus [13]. Similar alterations were revealed when sequencing mononuclear blood cells in individuals suffering from ARDS relative to healthy donors. Low counts of the major lymphocyte representatives, B cells and CD4 T cells, were reported [14].
The phenomenon referred to as “cytokine storm” that is characterized by high blood levels of pro-inflammatory cytokines (TNFα, IFNγ, IL6, IL1β) is a typical feature of ARDS. It has been shown that TNFα is involved in the development of fever, systemic inflammation enhancement, antimicrobial response activation, and increase in secretion of other proinflammatory cytokines (such as IL6). TNFα activates the NF-kB transcription factor that stimulates numerous genes involved in inflammatory response [15].
The lung is a barrier organ with the enormously developed system of protection against microorganisms. Macrophages are the first cells responding to foreign invasion. Two types of macrophages are distinguished in the lung: alveolar macrophages inhabiting the alveoli and interstitial macrophages found in the interstitial space [16]. Phenotype of the first is described as pro-inflammatory with high expression of IL10 and TGFβ [17]. Interstitial macrophages currently represent an extensively studied cell population [16]. Macrophages recruited from blood monocytes have a phenotype that is close to pro-inflammatory. These express large amounts of the co-stimulatory CD86 molecules essential for adequate antigenic signal transduction in complexes with the type 1 major histocompatibility complex (MHC I) molecules [9]. The disease outcome depends on the balance between two states of alveolar macrophages. Furthermore, it has been shown that the MHC IIhi memory cell population is formed of those in case of viral disease [18, 19]. Pro-inflammatory macrophages predominate over antiinflammatory cells in the exudative phase. Activation of the macrophage pattern recognition receptors leads to generation of the inflammasome, in which caspase-1 contributes to the IL1 and IL18 maturation. The disease progression can be severe, if the inflammasome maturation process is disturbed. In the ARDS proliferative phase, pro-inflammatory macrophages are replaced by the anti-inflammatory macrophages that remove cellular debris and release anti-inflammatory cytokines. It has been shown that abnormal efferocytosis can result in the prolonged inflammation observed in ARDS; moreover, at this stage excessive participation of anti-inflammatory macrophages in matrix reorganization processes can lead to chronic fibrosis and occlusion of blood vessels [20]. Thus, the increased counts of the CD86 and MHC II-positive cells we have revealed suggest that the lung macrophages are in the transitional state 48 h after the intranasal LPS administration: between pro-inflammatory and anti-inflammatory [18].
At the gene level it has been reported that intranasal administration of the LPS in a dose of 15 mg/kg of body weight results in alteration of expression of the genes encoding both pro- and anti-inflammatory cytokines (IL18 and IL10, respectively). Furthermore, the use of the higher dose significantly activated the anti-inflammatory immune response, which was indicated by high IL10 gene expression [21]. IL10 plays an ambiguous role in the ADRS pathogenesis: it can both contribute to the disease resolution through inhibition of pro-inflammatory cytokine (such as TNFα, IL1β, IL6, and IFNγ) secretion and complicate the disease due to the decreased stem cell differentiation into type II pneumocytes, thereby preventing lung recovery [22]. High IL10 expression can potentially be an indicator of macrophage polarization into the anti-inflammatory phenotype, which is consistent with high levels of MHC II-positive cells based on the flow cytometry results [17].
The IL18 pro-inflammatory cytokine that was first described as a factor inducing interferon IFNγ through effects of both innate and adaptive immune response is involved in protection against infectious agents and anti-tumor immunity. Activation of the innate immunity pattern recognition receptors (PRR) in the lung leads to activation of the NF-kb transcription factor in the epithelial and endothelial cells, as well as in the resident immune cells. NF-kb, in turn, contributes to the increase of pro-IL1β, pro-IL18, and pro-caspase-1, the mature forms of which that trigger the vicious circle of inflammation are formed in inflammasomes. IL18 is a potent chemoattractant for neutrophils, the increased counts of which in the lung represent the most important sign of the ARDS acute phase; this is consistent with our data on high IL18 gene expression after administration of the LPS dose of 15 mg/kg of body weight, as well as on high neutrophil counts in blood.
CONCLUSIONS
Intranasal administration of the LPS of the external wall of Gram-negative bacteria to Sprague-Dawley rats within 48 h leads to the development of processes typical for the ARDS exudative and proliferative phases: pulmonary edema, decreased lymphocyte counts and increased neutrophil counts in blood, increased serum levels of TNFα. LPS administration in a dose of 15 mg/kg leads to the rapid development of inflammation in the lung, and after 48 h, a significant activation of anti-inflammatory responses can be observed. Thus, the use of the O111:B4 LPS intranasal administration in a dose of 15 mg/kg of body weight to Sprague-Dawley rats can be used to model ARDS in order to assess the efficacy of drug therapy, including cell-based therapy, based on such indicators, as dry weight of the lung, counts of the CD86 and MHC II-positive cells in the lung homogenates, and IL10 and IL18 gene expression.
- Bos LDJ, Ware LB. Acute respiratory distress syndrome: causes, pathophysiology, and phenotypes. The Lancet. 2022; 400: 1145–56.
- Aeffner F, Bolon B, Davis IC. Mouse Models of Acute Respiratory Distress Syndrome. Toxicol Pathol. 2015; 43: 1074–92.
- Matute-Bello G, Frevert CW, Martin TR. Animal models of acute lung injury. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2008; 295: L379–99.
- Soloveva T, Davydova V, Krasikova I, Yermak I. Marine Compounds with Therapeutic Potential in Gram-Negative Sepsis. Mar Drugs. 2013; 11: 2216–29.
- Khadangi F, Forgues A-S, Tremblay-Pitre S, Dufour-Mailhot A, Henry C, Boucher M, et al. Intranasal versus intratracheal exposure to lipopolysaccharides in a murine model of acute respiratory distress syndrome. Sci Rep. 2021; 11: 7777.
- Kosyreva AM, Miroshnichenko EA, Tsvetkov IS, Lokhonina AV, Sentyabreva AV, Dzhalilova DSh, et al. Morphofunctional Characteristics of Lung Macrophages in Rats with Acute Respiratory Distress Syndrome. Bull Exp Biol Med. 2023; 175: 822–7.
- Available from: https://floreada.io/.
- Parker JC, Townsley MI. Evaluation of lung injury in rats and mice. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2004; 286: L231–46.
- Vishnyakova P, Poltavets A, Karpulevich E, Maznina A, Vtorushina V, Mikhaleva L, et al. The response of two polar monocyte subsets to inflammation. Biomedicine & Pharmacotherapy. 2021; 139: 111614.
- Chistiakov DA, Killingsworth MC, Myasoedova VA, Orekhov AN, Bobryshev YV. CD68/macrosialin: not just a histochemical marker. Laboratory Investigation. 2017; 97: 4–13.
- Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, et al. Macrophage Activation and Polarization: Nomenclature and Experimental Guidelines. Immunity. 2014;41:14–20.
- Qazi MR, Bogdanska J, Butenhoff JL, Nelson BD, DePierre JW, Abedi-Valugerdi M. High-dose, short-term exposure of mice to perfluorooctanesulfonate (PFOS) or perfluorooctanoate (PFOA) affects the number of circulating neutrophils differently, but enhances the inflammatory responses of macrophages to lipopolysaccharide (LPS) in a similar fashion. Toxicology. 2009; 262: 207–14.
- Chakraborty S, Zawieja SD, Wang W, Lee Y, Wang YJ, von der Weid P-Y, et al. Lipopolysaccharide modulates neutrophil recruitment and macrophage polarization on lymphatic vessels and impairs lymphatic function in rat mesentery. American Journal of Physiology-Heart and Circulatory Physiology. 2015; 309: H2042–57.
- Torres M, Casado G, Vigón L, Rodríguez-Mora S, Mateos E, Ramos-Martín F, et al. Changes in the immune response against SARS-CoV-2 in individuals with severe COVID-19 treated with high dose of vitamin D. Biomedicine & Pharmacotherapy. 2022; 150: 112965.
- Riyaz Tramboo S, Elkhalifa AME, Quibtiya S, Ali SI, Nazir Shah N, Taifa S, et al. The critical impacts of cytokine storms in respiratory disorders. Heliyon. 2024; 10: e29769.
- Hou F, Xiao K, Tang L, Xie L. Diversity of Macrophages in Lung Homeostasis and Diseases. Front Immunol. 2021; 12.
- Fakoya AO, Naeem A, Louisdon P, Histology. Alveolar Macrophages. Available from: https://www.ncbi.nlm.nih.gov/books/NBK513313/#:~:text=Alveolar%20macrophages%20produce%20anti%2Dinflammatory,processes%20and%20promote%20tissue%20repair.
- Morrell ED, Bhatraju PK, Mikacenic CR, Radella F, Manicone AM, Stapleton RD, et al. Alveolar Macrophage Transcriptional Programs Are Associated with Outcomes in Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med. 2019; 200: 732–41.
- Yao Y, Jeyanathan M, Haddadi S, Barra NG, Vaseghi-Shanjani M, Damjanovic D, et al. Induction of Autonomous Memory Alveolar Macrophages Requires T Cell Help and Is Critical to Trained Immunity. Cell. 2018; 175: 1634–50.e17.
- Mahida RY, Scott A, Parekh D, Lugg ST, Hardy RS, Lavery GG, et al. Acute respiratory distress syndrome is associated with impaired alveolar macrophage efferocytosis. European Respiratory Journal. 2021; 58: 2100829.
- Couper KN, Blount DG, Riley EM. IL-10: The Master Regulator of Immunity to Infection. The Journal of Immunology. 2008; 180: 5771–7.
- Sun Z, Chen A, Fang H, Sun D, Huang M, Cheng E, et al. B cell-derived IL-10 promotes the resolution of lipopolysaccharide-induced acute lung injury. Cell Death Dis. 2023; 14: 418.