This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (CC BY).
ORIGINAL RESEARCH
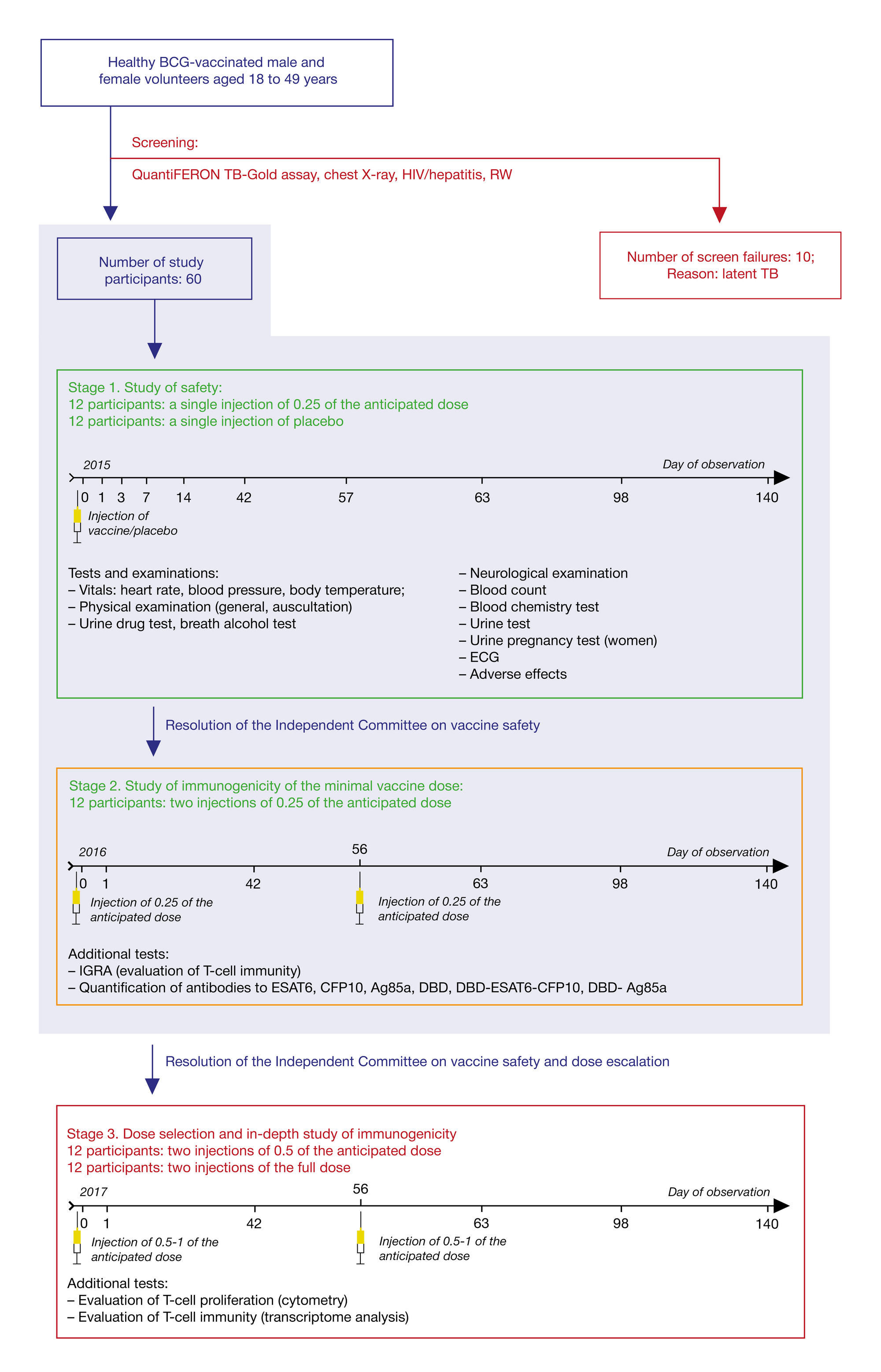
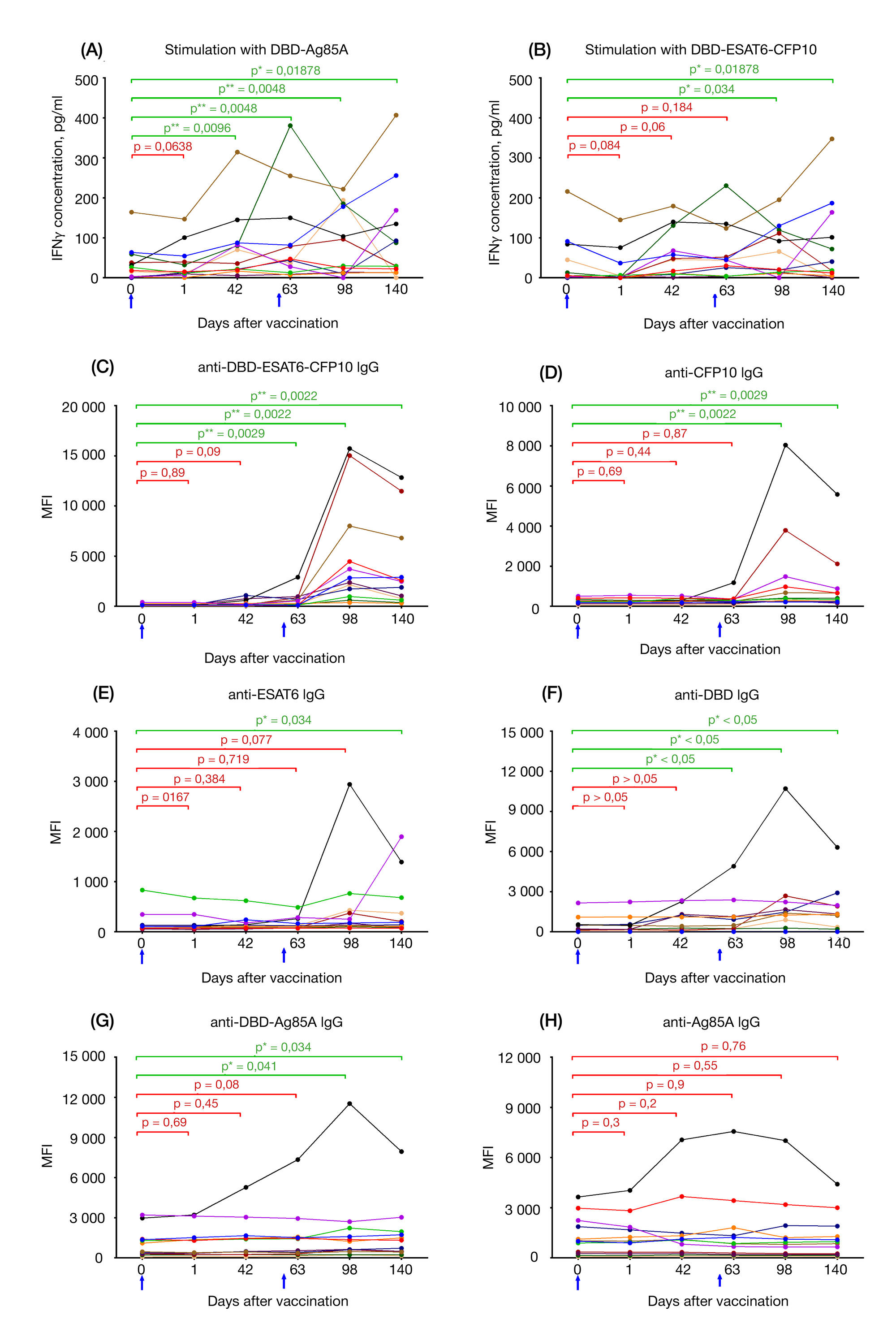
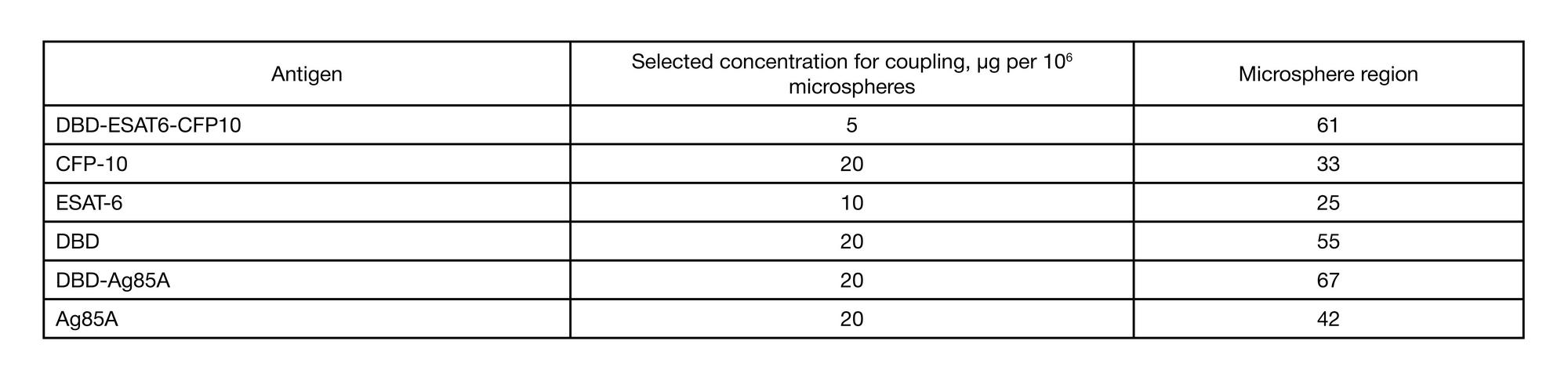
Immunological memory formed in response to administration of GamTBvac recombinant tuberculosis vaccine candidate: clinical trials in healthy volunteers
1 Laboratory of Translational Biomedicine,N. F. Gamaleya Federal Research Centre for Epidemiology and Microbiology, Moscow, Russia
2 Institute of Molecular Medicine,I. M. Sechenov First Moscow State Medical University, Moscow, Russia
3 Department of Virology, Faculty of Biology,Lomonosov Moscow State University, Moscow
Correspondence should be addressed: Denis A. Kleymenov
ul. Gamalei, d. 18, Moscow, Russia, 123098; ur.relbmar@tel00001
Funding: this work was supported by the Russian Ministry of Health and the Federal Agency for Scientific Organizations (state task no. 0574-2014-0023)
Contribution of the authors to this work: Kleymenov DA — analysis of literature, research planning, research preparation, data collection, analysis, and interpreatation, drafting of a manuscript, writing of a final version of a manuscript; Mazunina EP — analysis of literature, research planning, research preparation, data collection, analysis, and interpretation, drafting of a manuscript; Lunin VG — analysis of literature, research planning, research preparation, drafting of a manuscript; Koptev EYu, Manuilov VA — analysis of literature, research preparation, drafting of a manuscript; Gushchin VA — analysis of literature, research planning, research preparation, data analysis and interpretation, drafting of a manuscript, writing of a final version of a manuscript; Tkachuk AP — analysis of literature, research planning, research preparation, drafting of a manuscript, critical analysis of a manuscript.