This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (CC BY).
METHOD
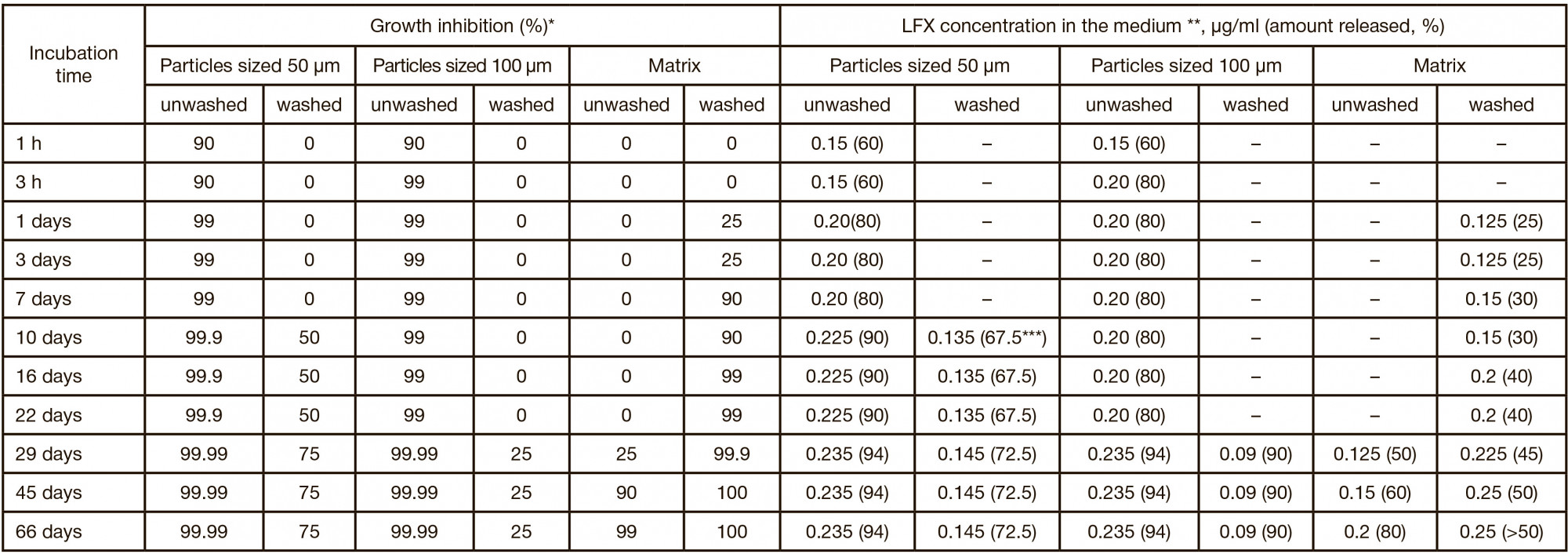
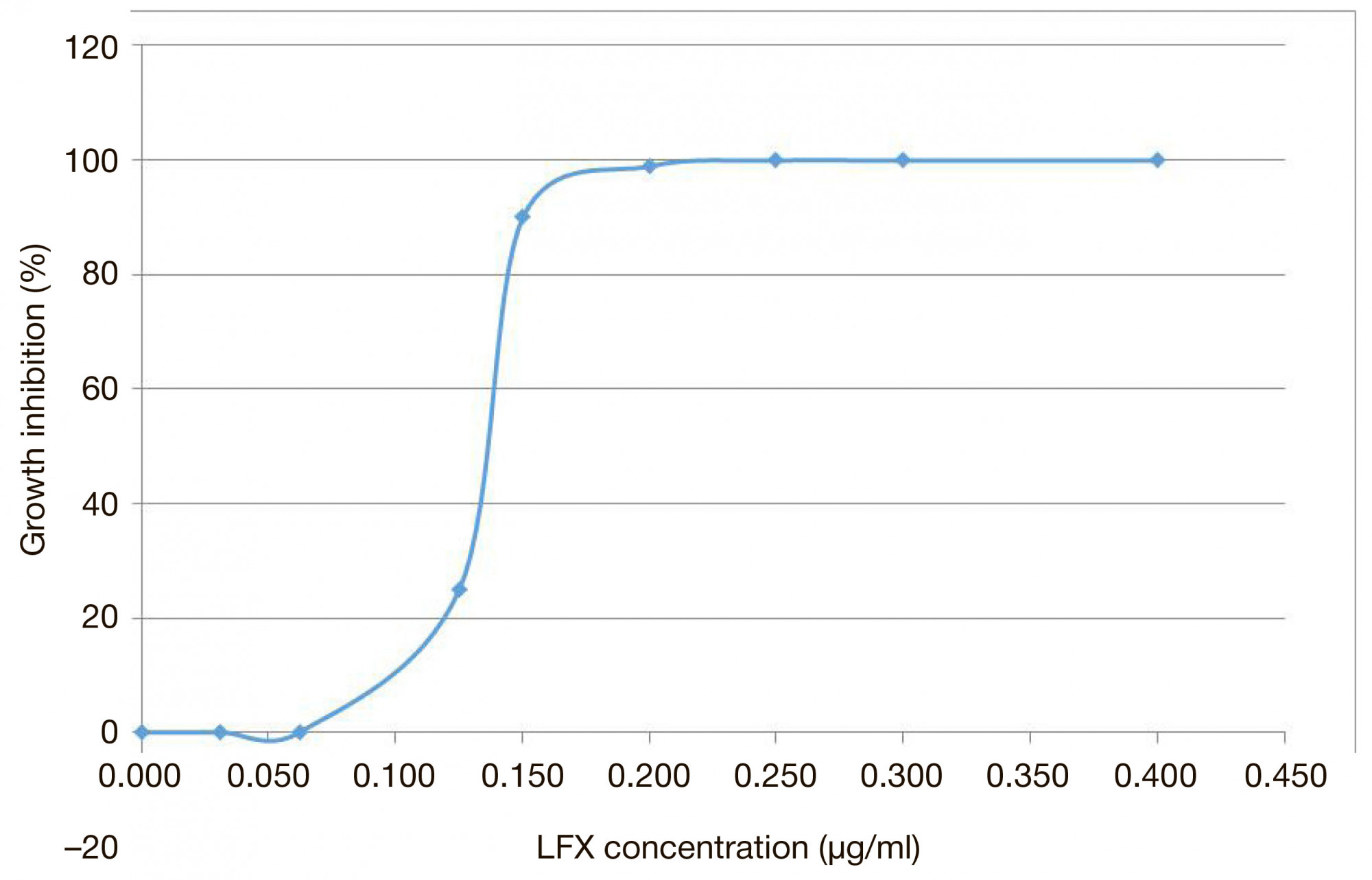
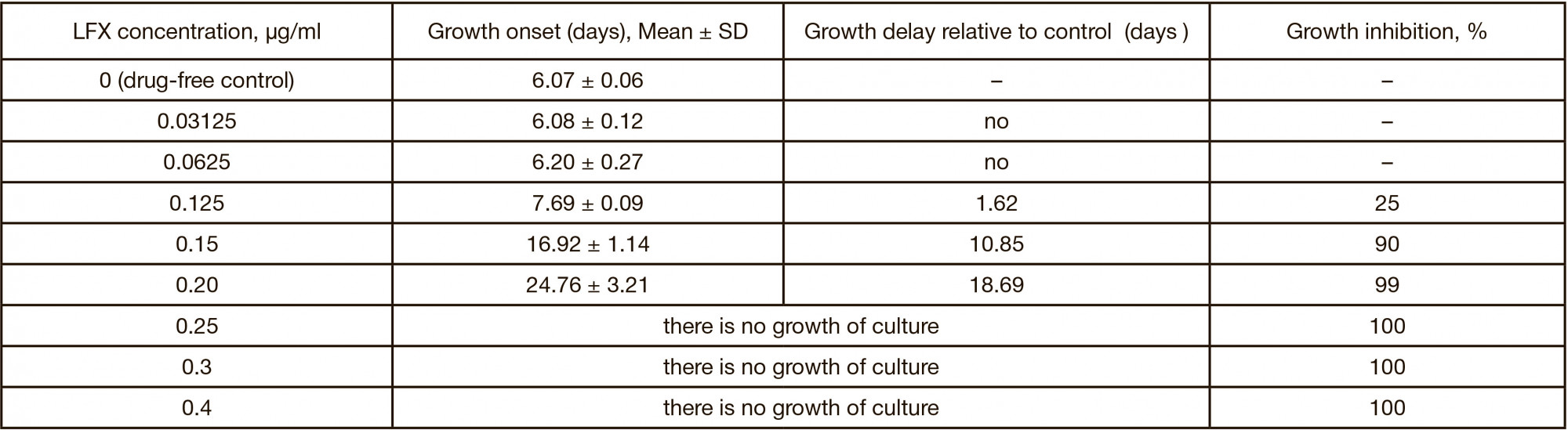
New in vitro model to evaluate kinetics of antimycobacterial drug release from bioresorbable polymeric carriers
1 Laboratory of Biotechnology, Central Tuberculosis Research Institute, Moscow, Russia
2 Institute of Photon Technologies FSRC ‘Crystallography and Photonics’ RAS, Moscow, Russia
Correspondence should be addressed: Sophia Nikolayevna Andreevskaya
Yauzskaya alleya, d. 2, str. 1A., Moscow, 107564; ur.liam@aifosdna
Funding: the study was supported by the Russian Ministry of Science and Higher Education and carried out as part of the State Assignment for FSRC “Crystallography and Photonics” RAS (developing an SCF-based method for creating bioactive matrices), as part of the State Assignment № 0515-2019-0015 (Formation of resistance to antimycobacterial drugs in mycobacteria and somatic cells) for the Central Tuberculosis Research Institute (evaluation of the bacteriostatic activity of different levofloxacin concentrations). The study was also supported by the Russian Foundation for Basic Research (Project № 18-29-06062 mk: development of sustained-release therapeutic formulations and an in vitro model for the evaluation of their efficacy).
Compliance with ethical standards: manipulations with virulent strains of M. tuberculosis were conducted in compliance with the safety guidelines for the experiments involving Risk Group III–IV pathogens, infectious agents and utilization of medical waste specified in sanitary regulations 1.3.2322-08, 1.3.2518-09, 1.3.2885-11, and 2.1.7.2790-10.
Author contribution: Andreevskaya SN — interpretation of study results, manuscript preparation; Smirnova TG — modeling of conditions for levofloxacin release from its carriers; discussion of study results; Antonov EN — preparation of matrices; discussion of study results; Chernousova LN, Ergeshov AE — study design; discussion of study results; Bogorodsky SE — preparation of microparticles; discussion of study results; Larionova EE — literature analysis; discussion of study results; Popov VK — method for antibiotic encapsulation into polymeric carriers; discussion of study results.