This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (CC BY).
ORIGINAL RESEARCH
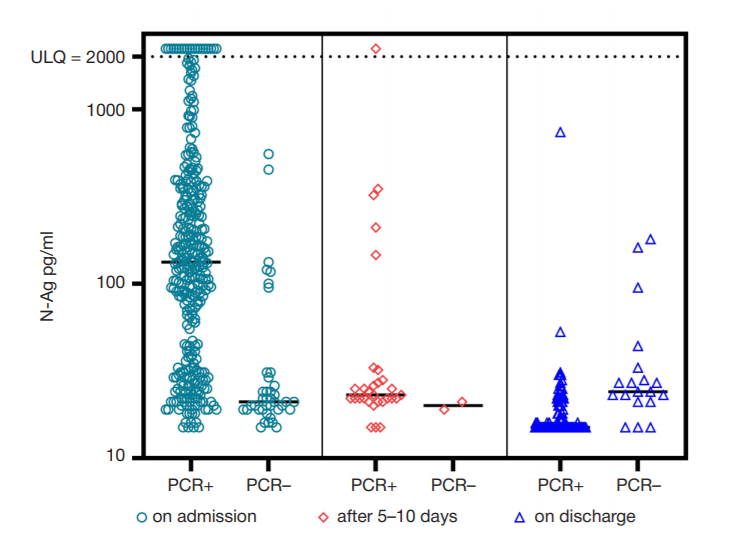
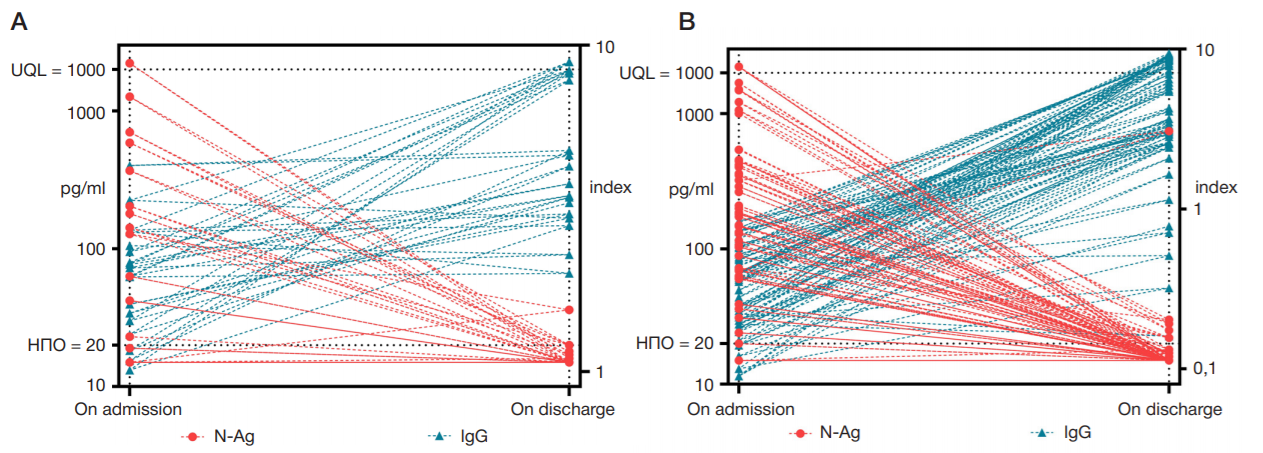
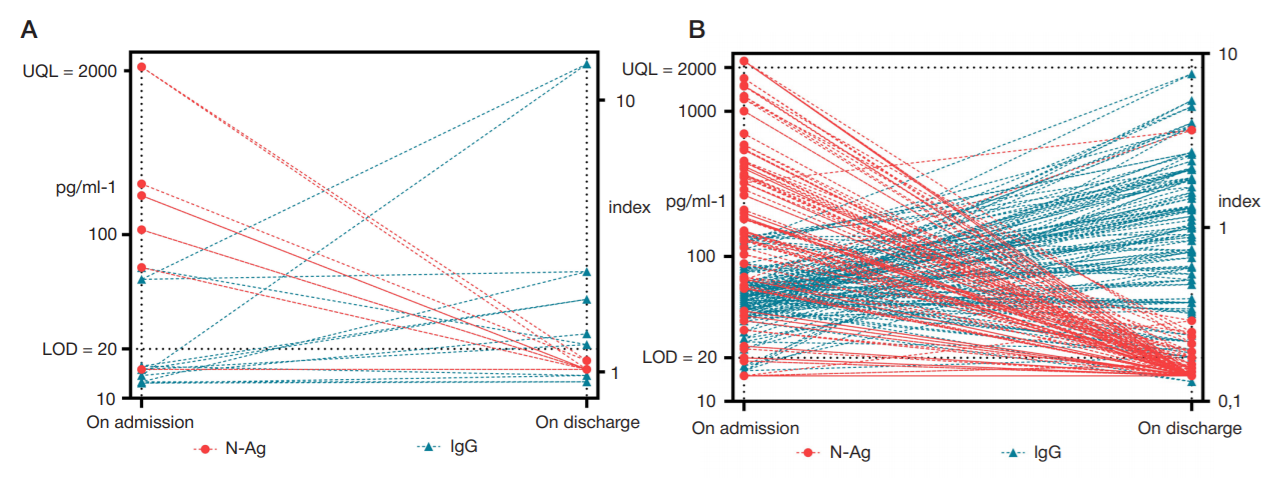
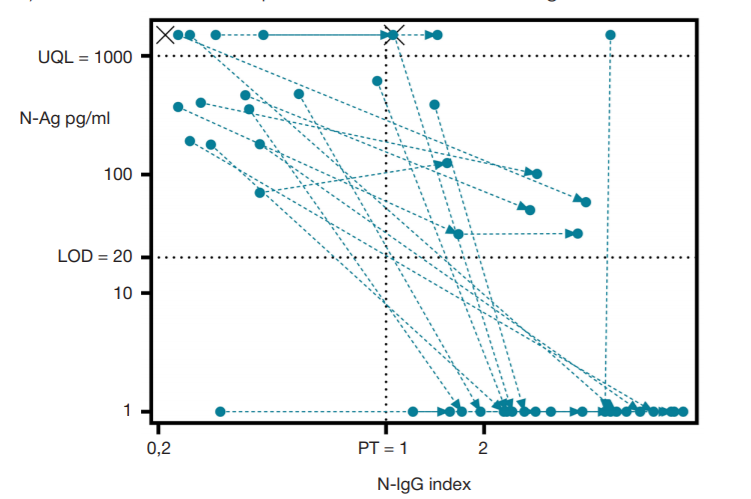
The importance of determining SARS-CoV-2 N-Ag serodiagnostics for the management of COVID-19 pneumonia in hospital settings
1 XEMA Co. Ltd., Moscow, Russia
2 Federal Center for Brain Research and Neurotechnologies of FMBA, Moscow, Russia
3 Center for Precision Genome Editing and Genetic Technologies for Biomedicine, Pirogov Russian National Research Medical University, Moscow, Russia
Funding: this work was partially supported by a grant № 075-15-2019-1789 from the Ministry of Science and Higher Education of the Russian Federation allocated to the Center for Precision Genome Editing and Genetic Technologies for Biomedicine.
Acknowledgement: we acknowledge Natalia Usman for helpful discussions
Author contribution: Lebedin YS designed the study, performed the sampling, conducted molecular studies and drafted the manuscript, Lyang OV and Galstyan AG performed the clinical examinations and sampling, conducted biochemical and molecular studies, Panteleeva AV conducted molecular studies and drafted the manuscript, Belousov VV and Rebrikov DV designed the study and drafted the manuscript. All authors read and approved the final version of the manuscript.
Compliance with ethical standards: the study protocol was reviewed and approved by the Local Ethics Committee at the Pirogov Russian State Medical University (Protocol № 2020/07 dated March 16, 2020,); the study was conducted in accordance with the Declaration of Helsinki.