This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (CC BY).
ORIGINAL RESEARCH
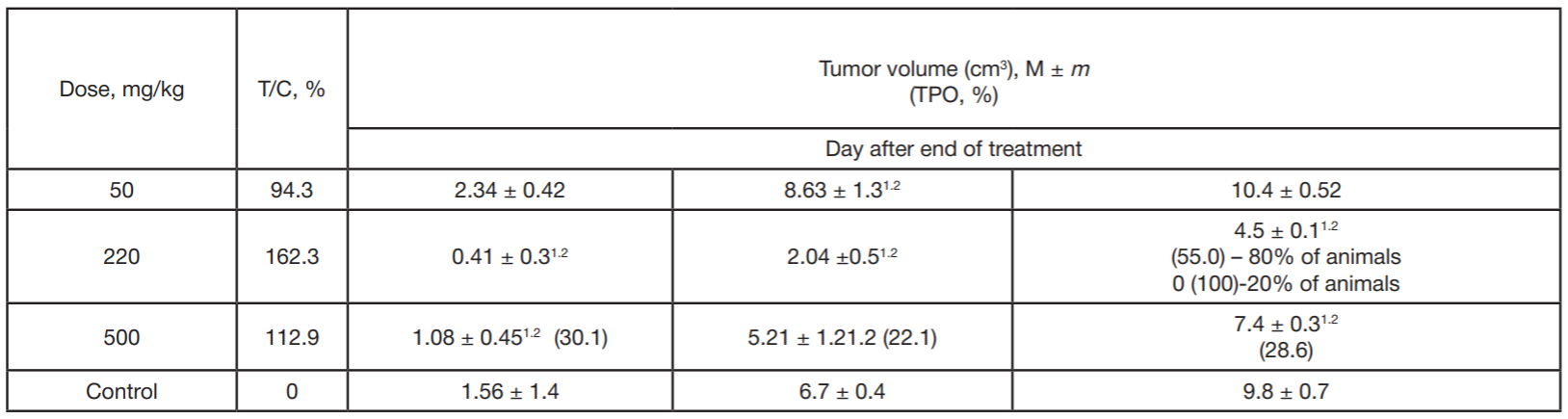
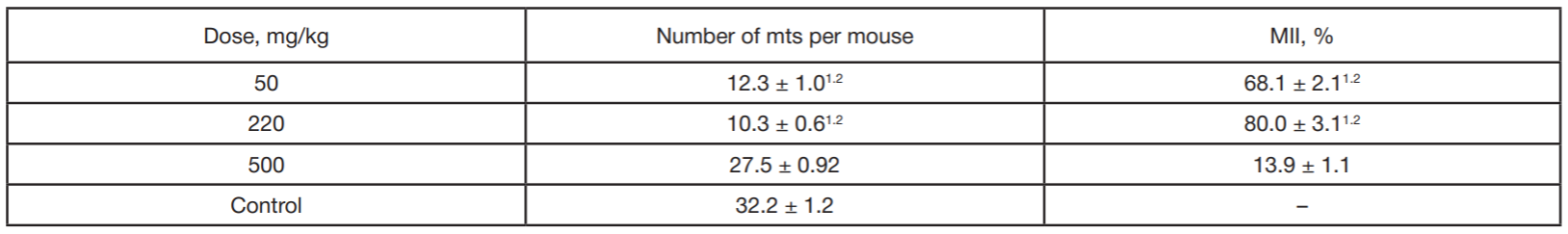
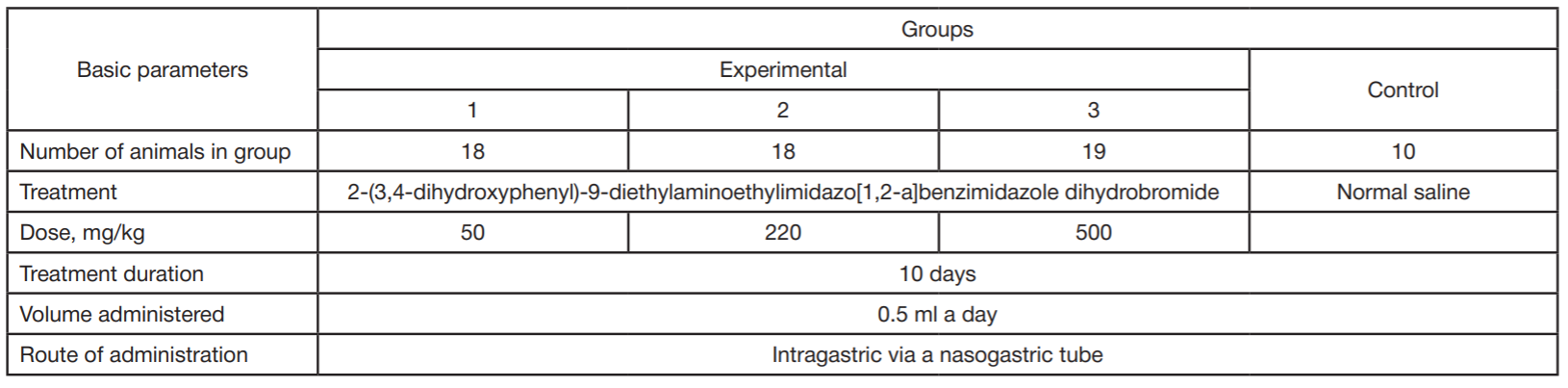
Benzimidazole derivative as antitumor drug against experimentally induced lung carcinoma
1 National Medical Research Center of Oncology, Rostov-on-Don, Russia
2 Rostov State Medical University, Rostov-on-Don, Russia
3 Research Institute for Physical and Organic Chemistry of Southern Federal University, Rostov-on-Don, Russia
Correspondence should be addressed: Ekaterina A. Lukbanova
Azovskaya, 163, 346783, Azov; ur.xednay@ajaksramas.aytak
Funding: synthesis of the tested compound was supported by the Russian Ministry of Science and Higher Education under the state assignment for the Southern Federal University, 2020, Project FENW-2020-0031 (0852-2020-0031). In vivo experiments were part of the state assignment Study of antitumor activity of pharmacological substances in vivo and in vitro (121031100253-3).
Author contributions: Komarova EF proposed the design, conducted the experiment and wrote the draft version of the manuscript; Zhukovskaya ON synthesized the tested compound and edited the manuscript; Lukbanova EA conducted the experiment and contributed to writing the manuscript; Yengibaryan MA edited the manuscript; Vashenko LN proposed the concept and design for the study, edited the manuscript; Kharagezov DA contributed to writing the manuscript; Pozdnyakova VV performed statistical analysis; Ushakova ND proposed the concept and design for the study; Shatova YuS prepared the list of references and edited the manuscript; Przhedetsky YuV performed technical editing.
Compliance with ethical standards: the study was approved by the Ethics Committee of National Medical Research Center for Oncology (Protocol № 18 dated September 10, 2015); the experiment complied with the European Convention for the Protection of Vertebrate Animals used for Experimental and other Scientific Purposes.