This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (CC BY).
ORIGINAL RESEARCH
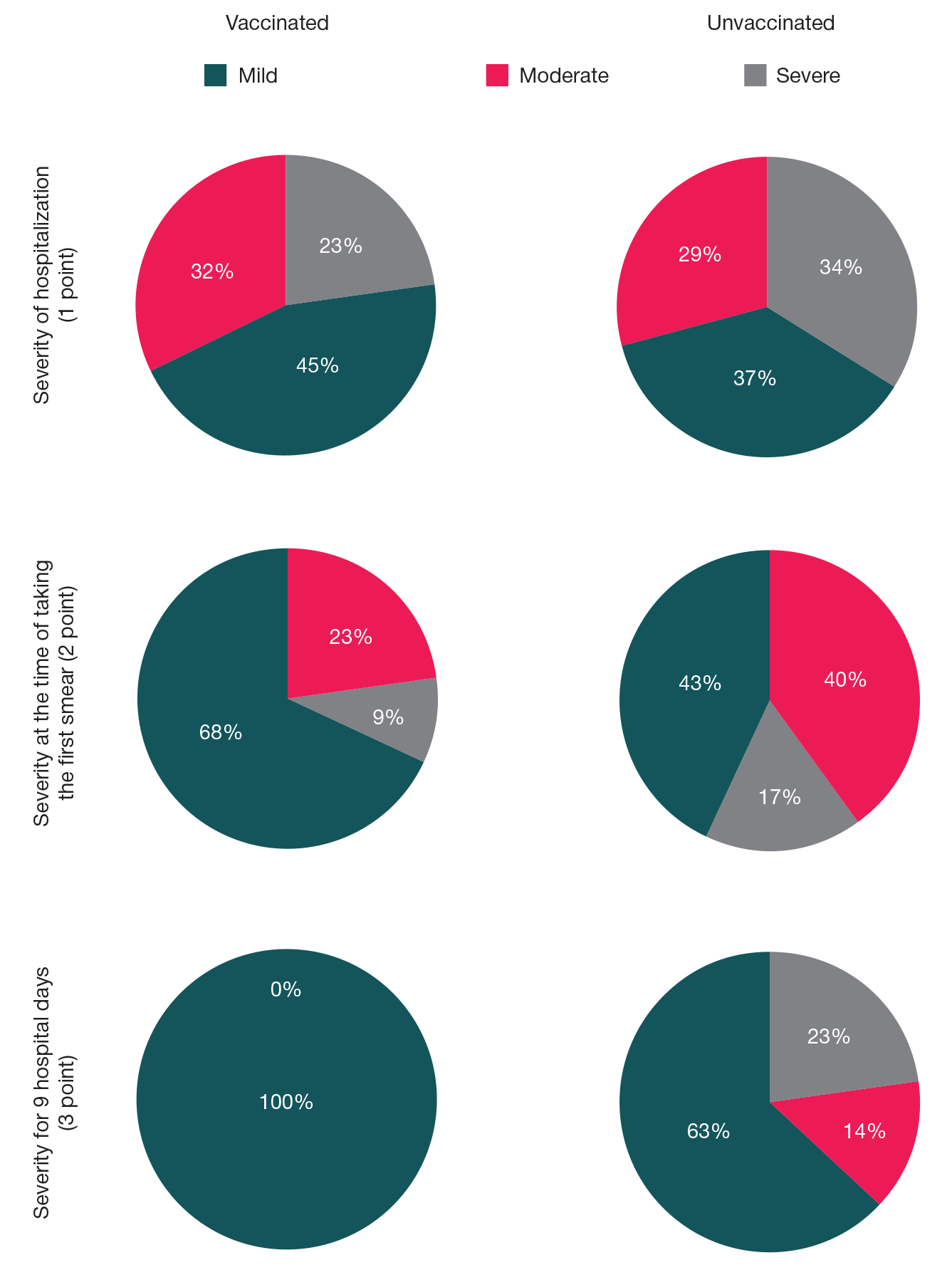
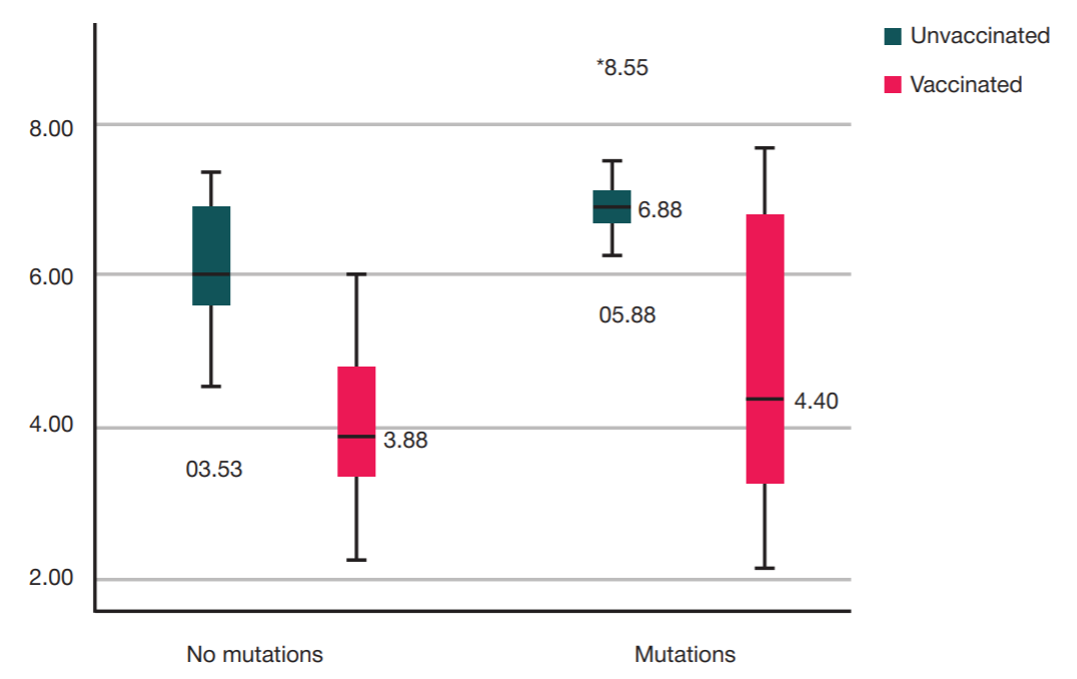
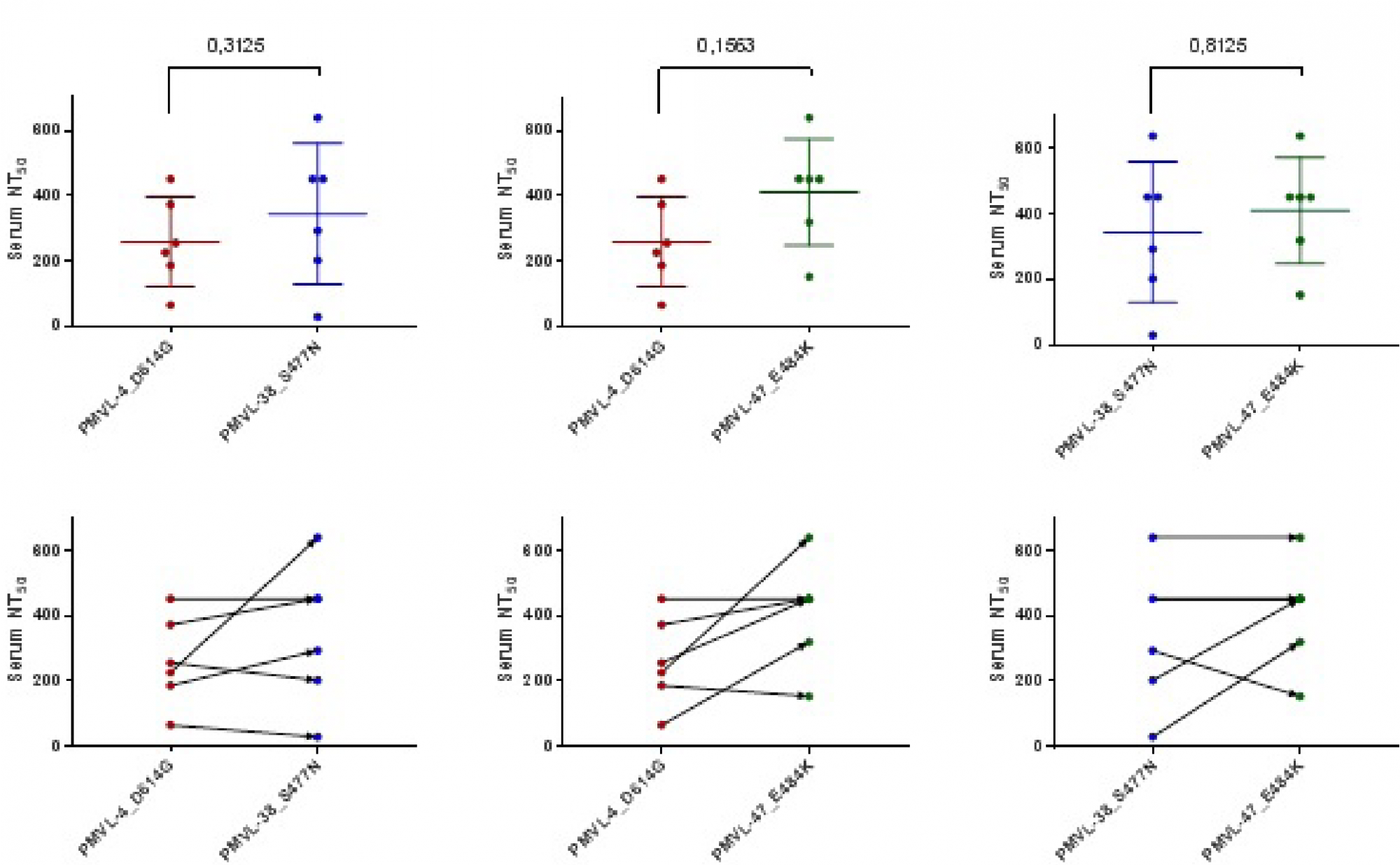
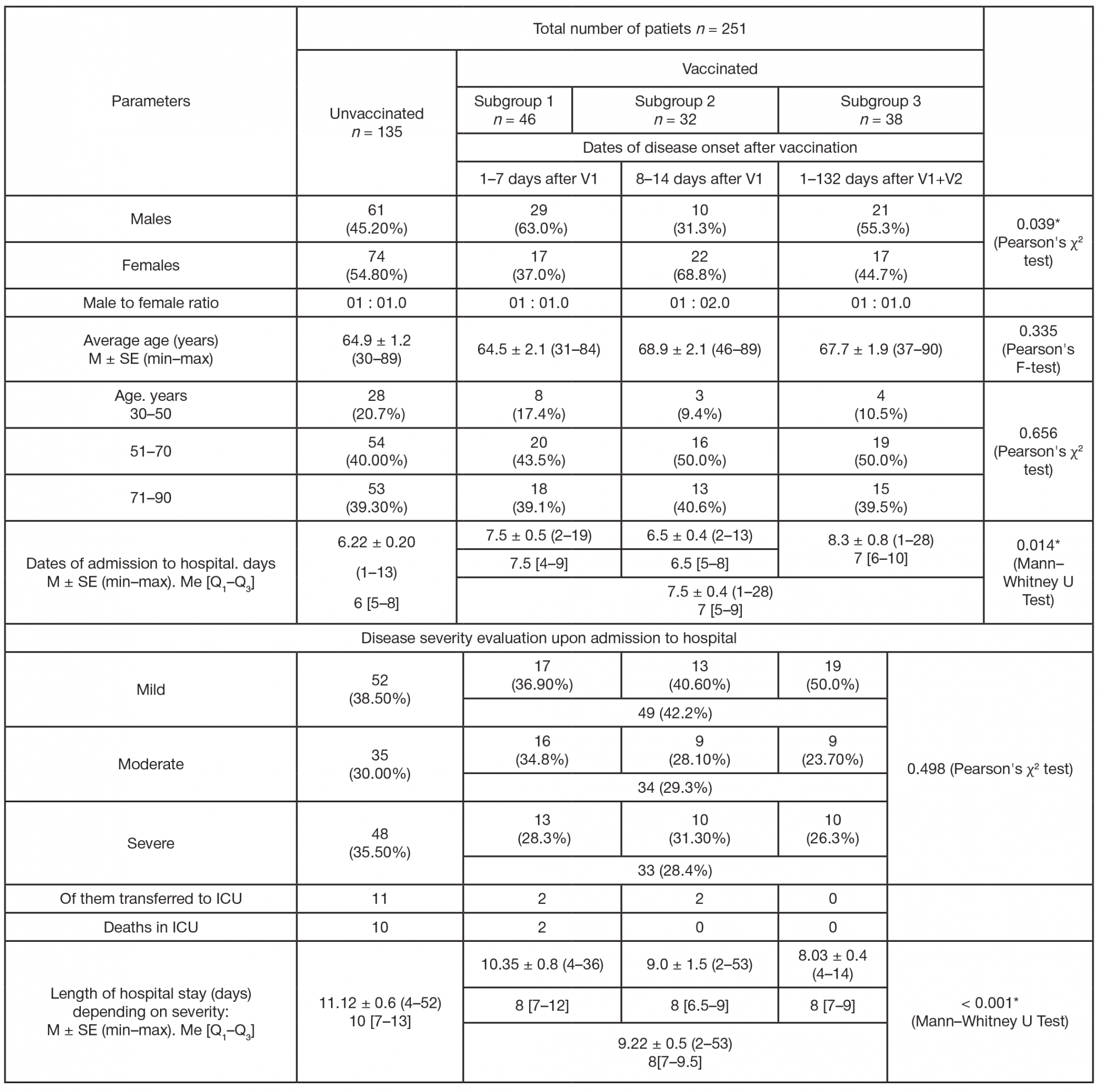
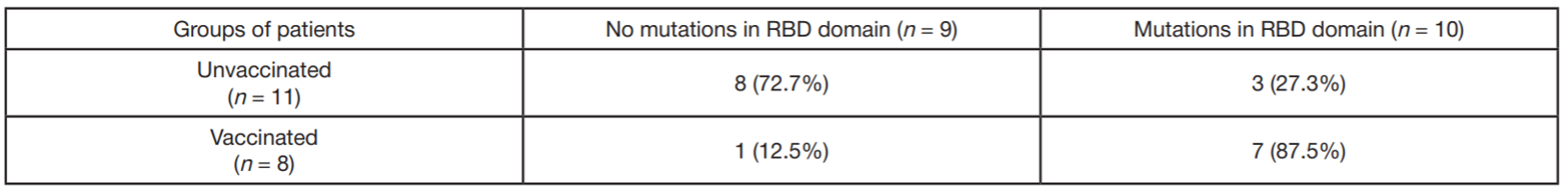
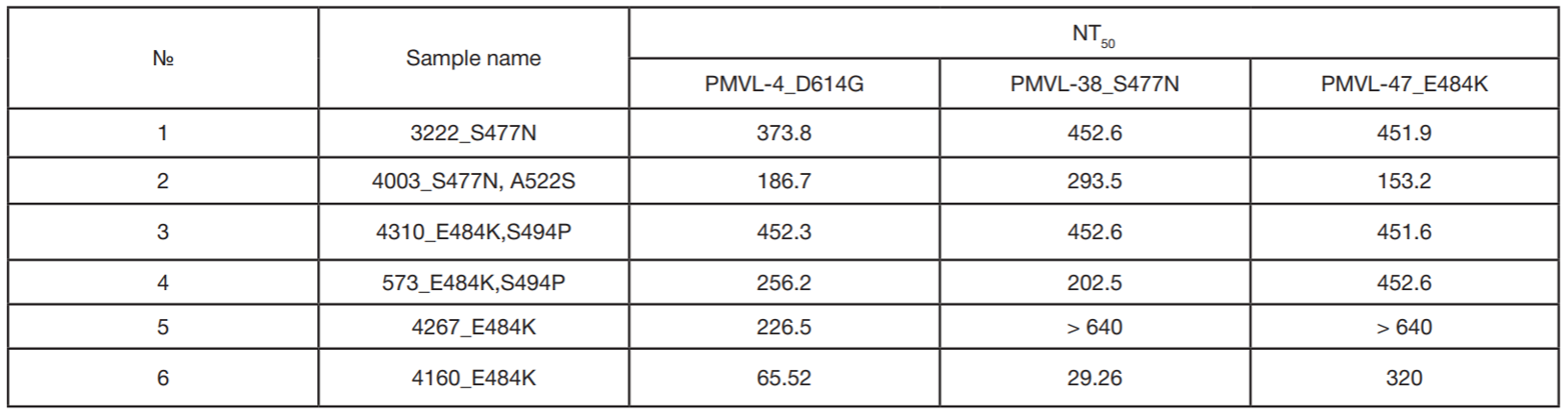
Assessment of COVID-19 clinical course in patients vaccinated with Spitnik V, SARS-CoV-2 S protein RBD domain variation and serum virus neutralizing activity
1 Gamaleya National Research Center for Epidemiology and Microbiology, Moscow, Russia
2 Peoples’ Friendship University of Russia, Moscow, Russia
3 Infectious Clinical Hospital № 1, Moscow, Russia
4 Lomonosov Moscow State University, Moscow, Russia
Correspondence should be addressed: Ludmila V. Kolobukhina
Gamaleya St. 18, Moscow, 123098; ur.xednay@anihcubolokl
Acknowledgements: we would like to thank Antipyat NA, Deputy Chief Physician for Medical Affairs, and Bazarova MV, PhD, Deputy Chief Physician for Sanitary and Epidemiological Issues, Infectious Clinical Hospital № 1, for the study management and support.
Author contributions: Kolobukhina LV — study proponent, design, manuscript writing, clinical research management; Burgasova OA — literature analysis, manuscript writing and editing, clinical research data processing; Kruzhkova IS — clinical observation, literature analysis, processing of the results; Bakalin VV — clinical observation, clinical and laboratory data processing; Generalova LV — clinical data processing; Shagaev AV — monitoring of infected individuals after vaccination; Ogarkova DA — statistical analysis; Nikiforova MA — coordination of virological studies, virus isolation and VNA; Vasina DV — ELISA data processing, coordination of immunological studies; Gushchin VA — study concept, molecular biological and virological research management; Smetanina SV — clinical research general management.
Compliance with ethical standards: the study was approved by the Ethics Committee of Moscow Infectious Clinical Hospital (protocol № 11/А dated November 16, 2020). The informed consent was submitted by all patients.