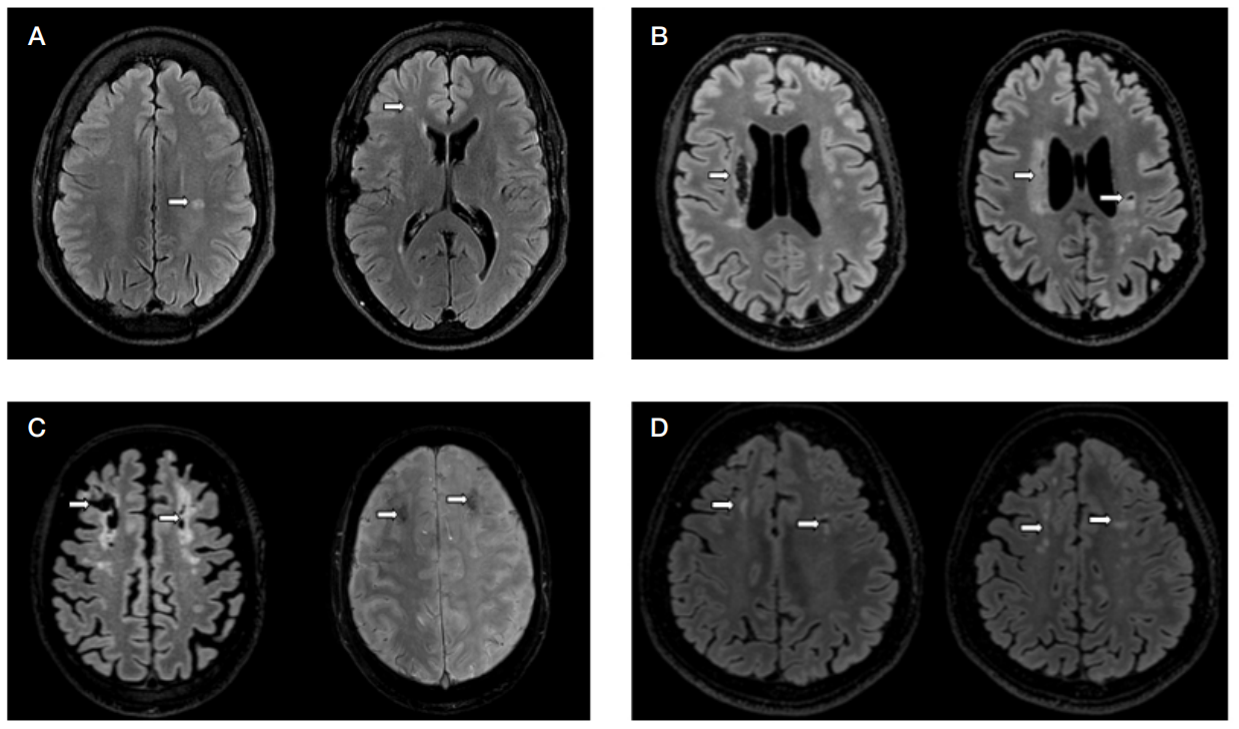
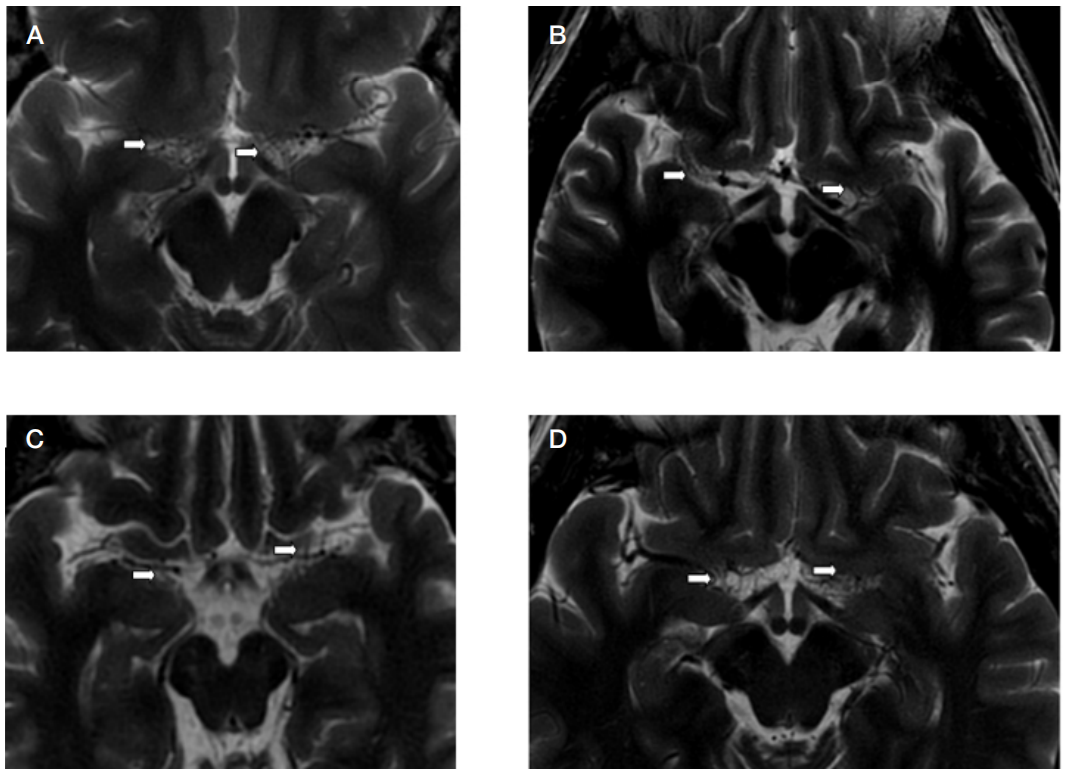
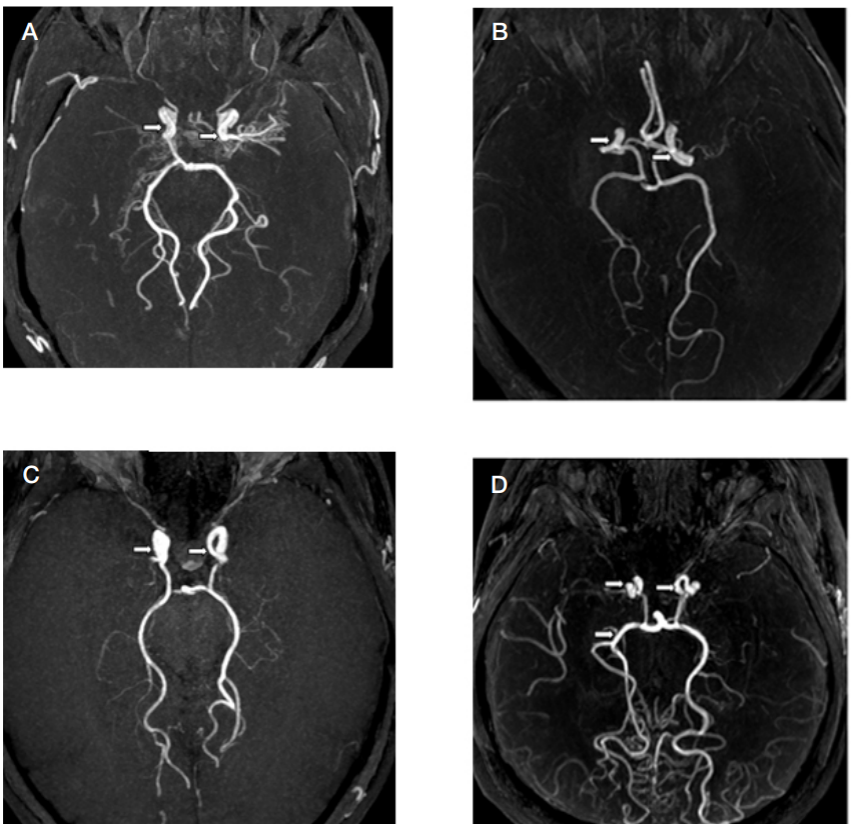
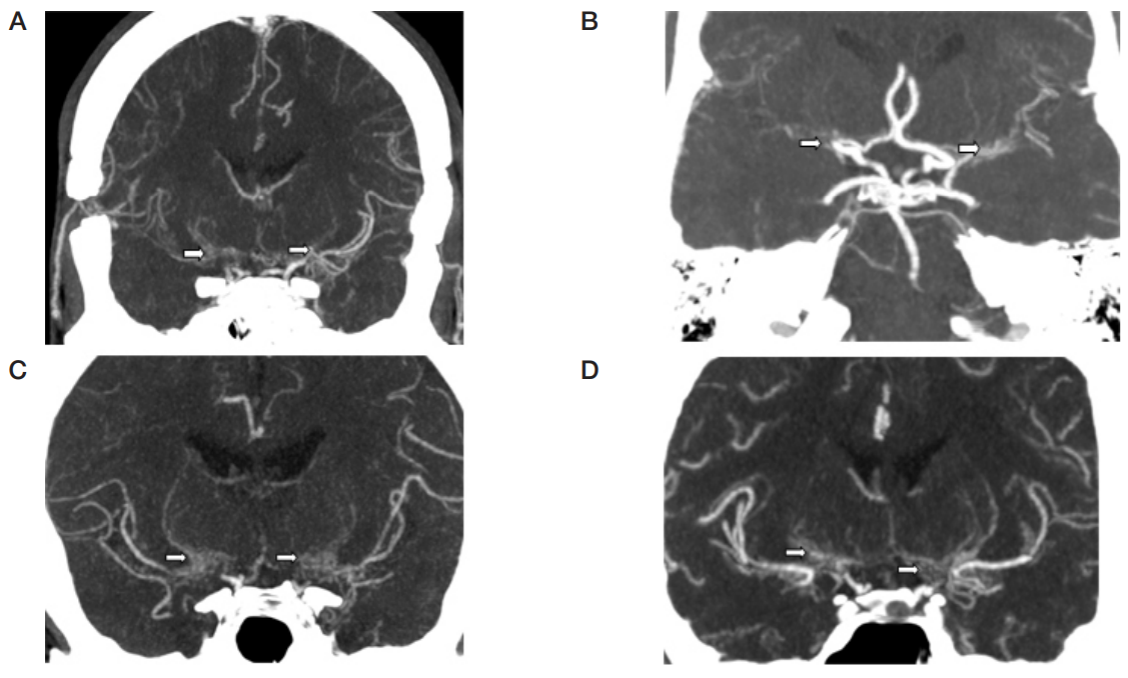
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (CC BY).
CLINICAL CASE
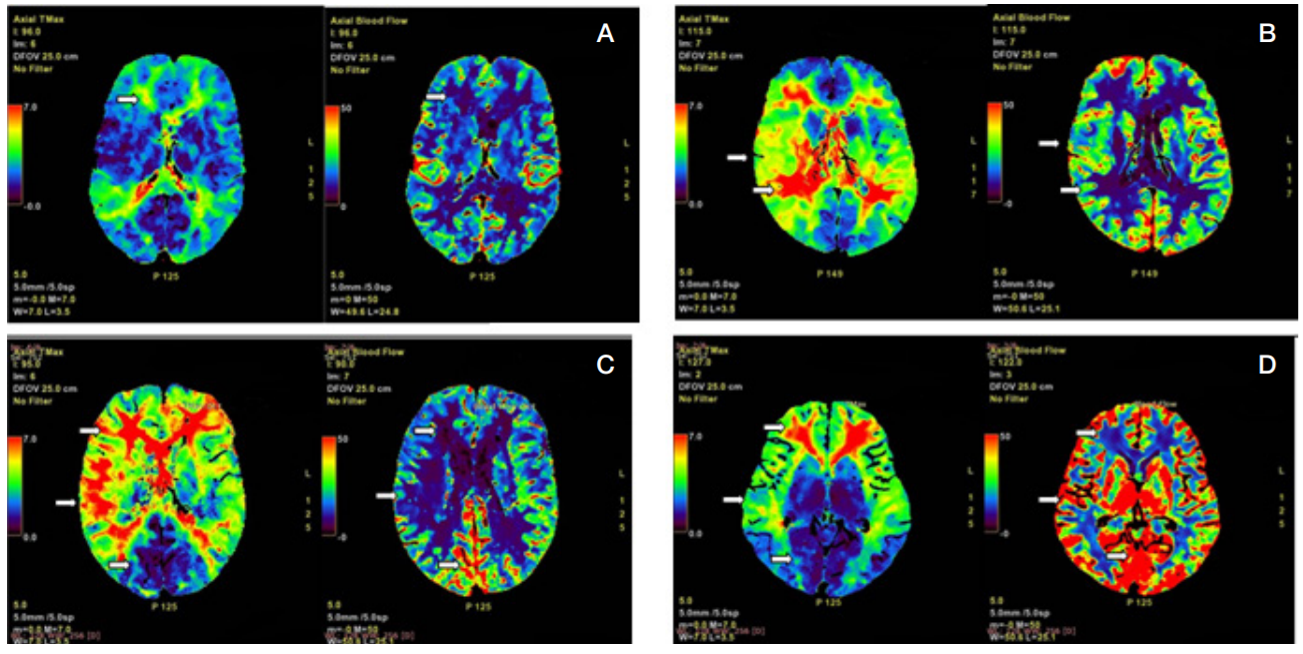
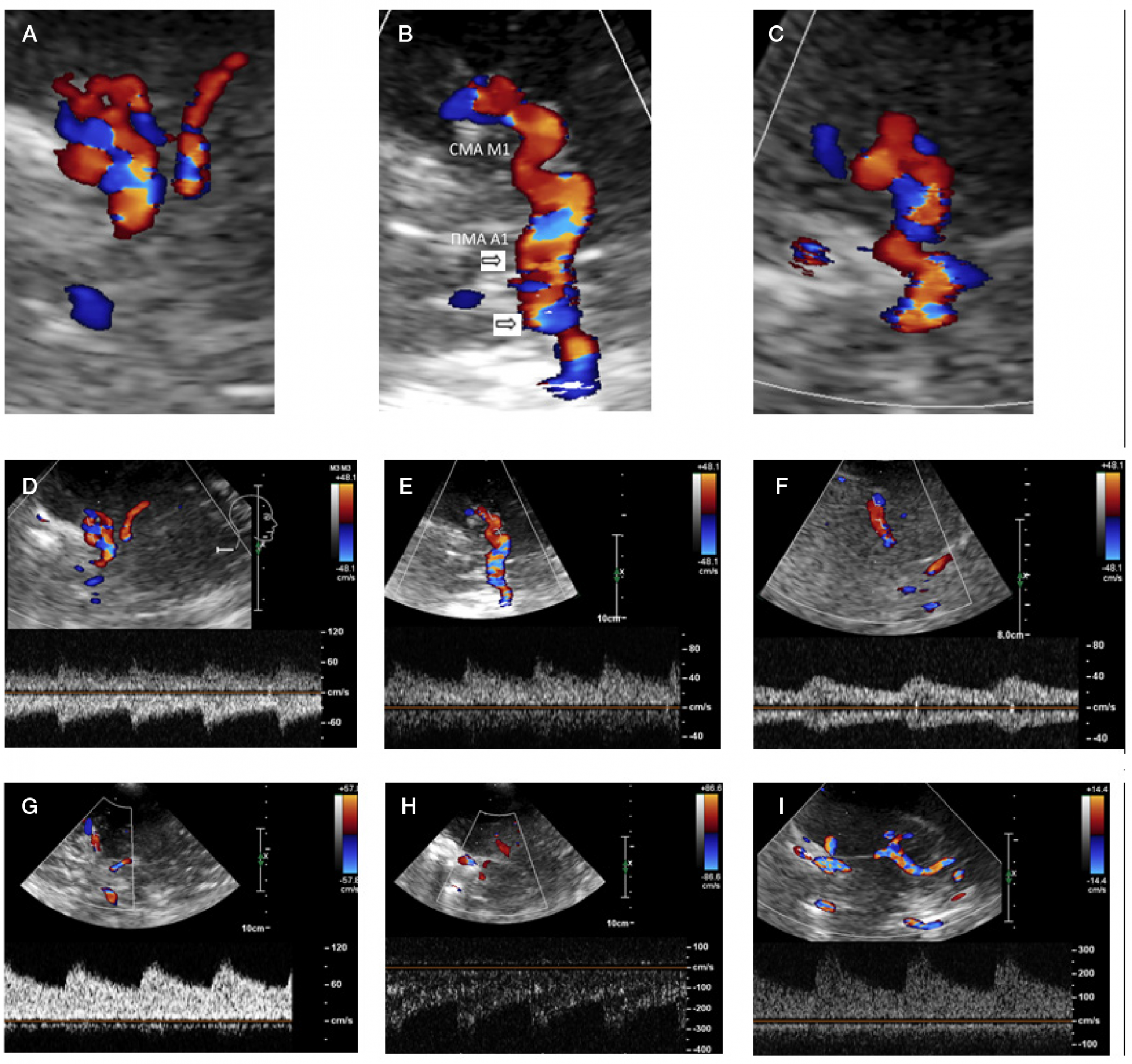
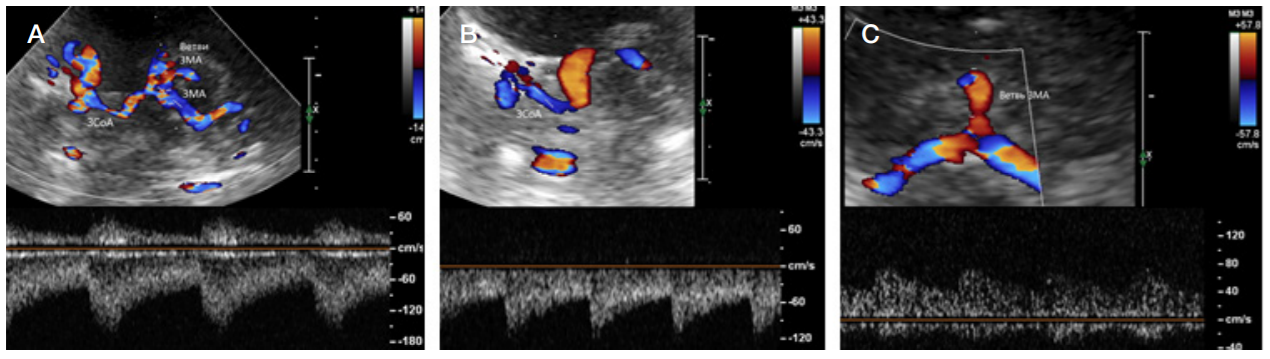
Moyamoya disease as a possible cause of ischemic stroke in adult patients
1 Federal Center of Brain Research and Neurotechnologies, Moscow, Russia
2 Russian Medical Academy of Continuous Professional Education, Moscow, Russia
Correspondence should be addressed: Anastasia Yu. Vishnyakova
Ostrovityanova 1, str. 10, Moscow, 117997; ur.xednay@uahsiv
Funding: the study was part of the State Assignment 056-00171-19-01. Topic ID: АААА-А19-119042590018-0 (March 29, 2019).
Author contribution: Vishnyakova AYu — literature analysis, imaging, data analysis and interpretation, manuscript preparation; Rostovtseva TM — imaging, data analysis and interpretation, figures; Kovrazhkina EA — clinical examination; Golovin DA — imaging; Gubsky IL — data analysis and interpretation; Lelyuk SE — manuscript editing; Lelyuk VG — study concept, manuscript editing.
Compliance with ethical standards: the study was approved by the Ethics Committee of FSBI Federal Center of Brain Research and Neurotechnologies of the Federal Medical Biological Agency (Protocol dated October 4, 2021). All patients gave informed consent to participate in the study.