This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (CC BY).
ORIGINAL RESEARCH
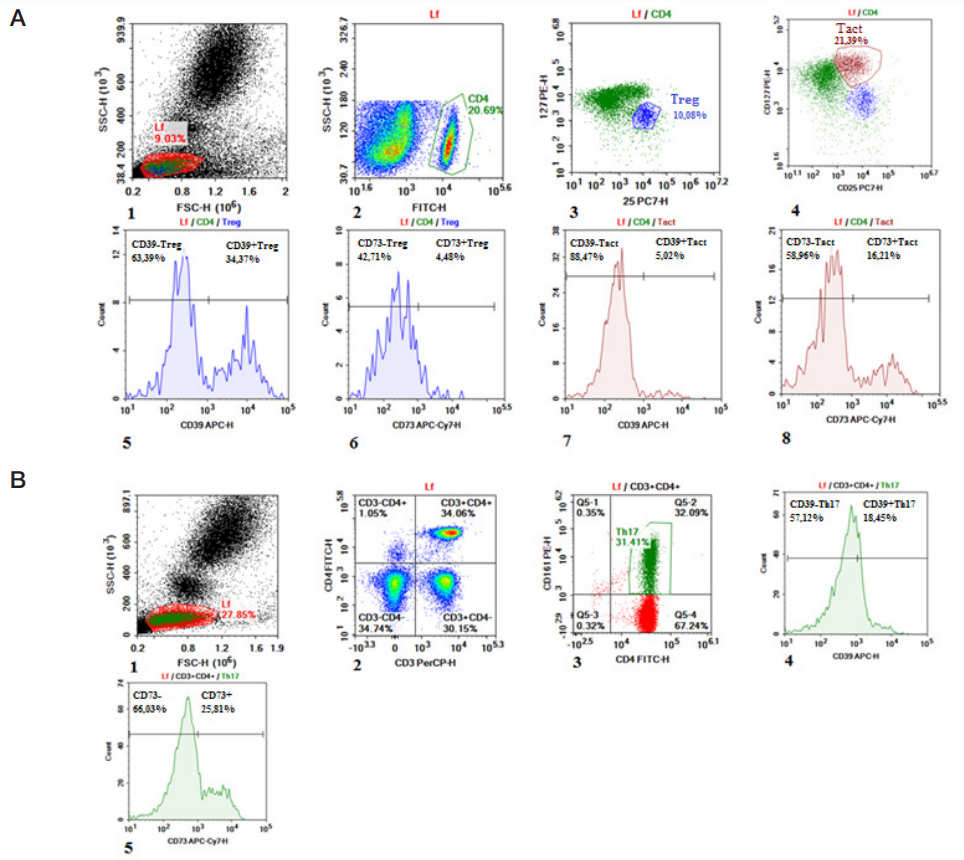
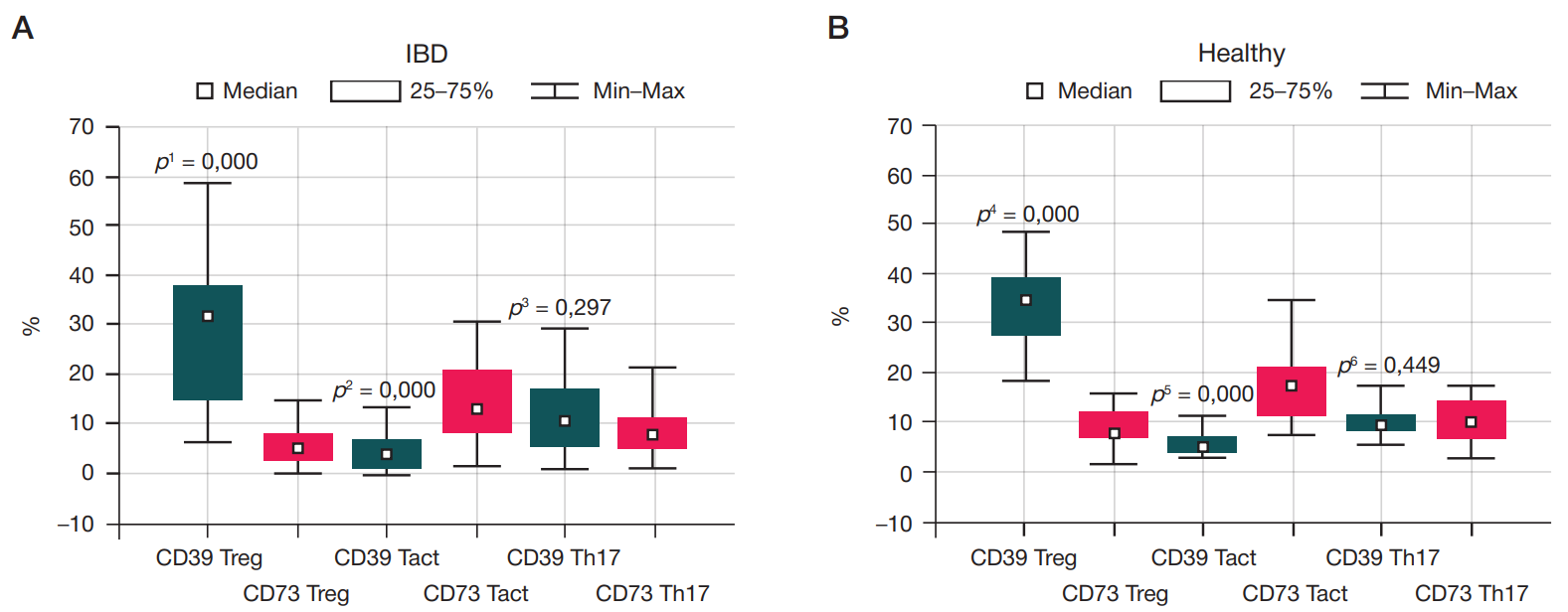
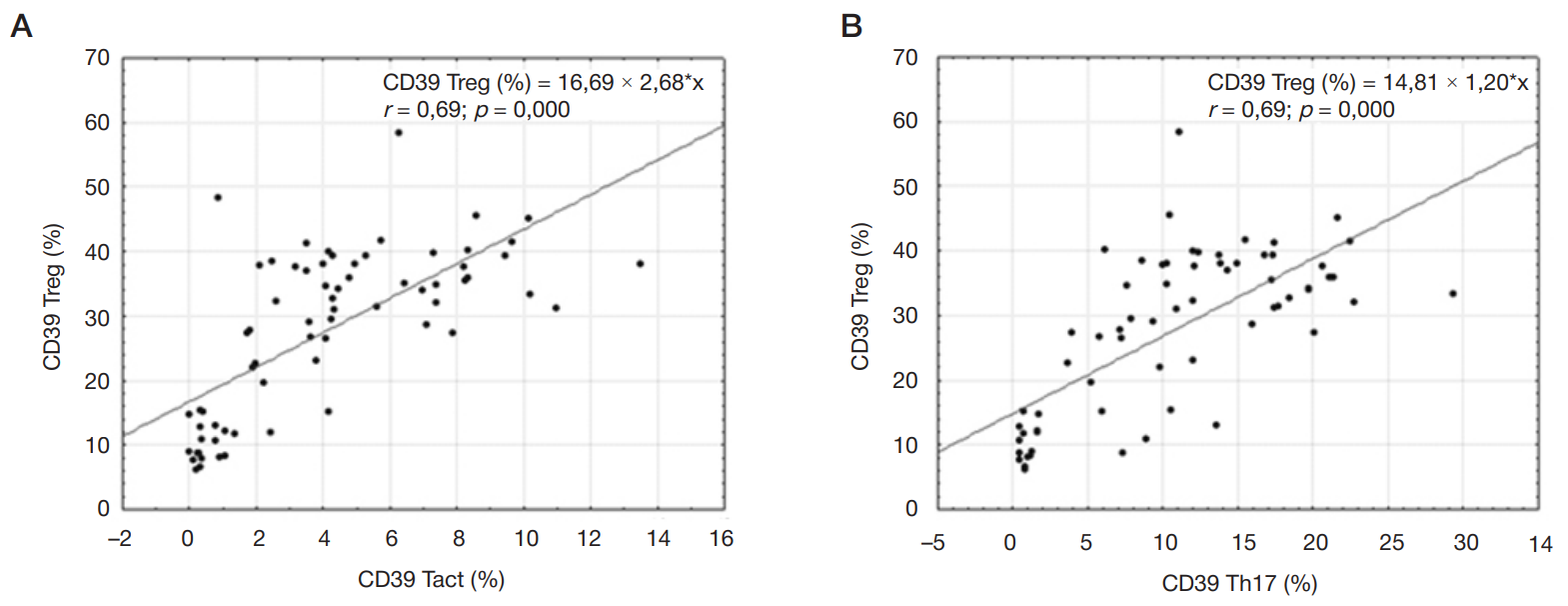
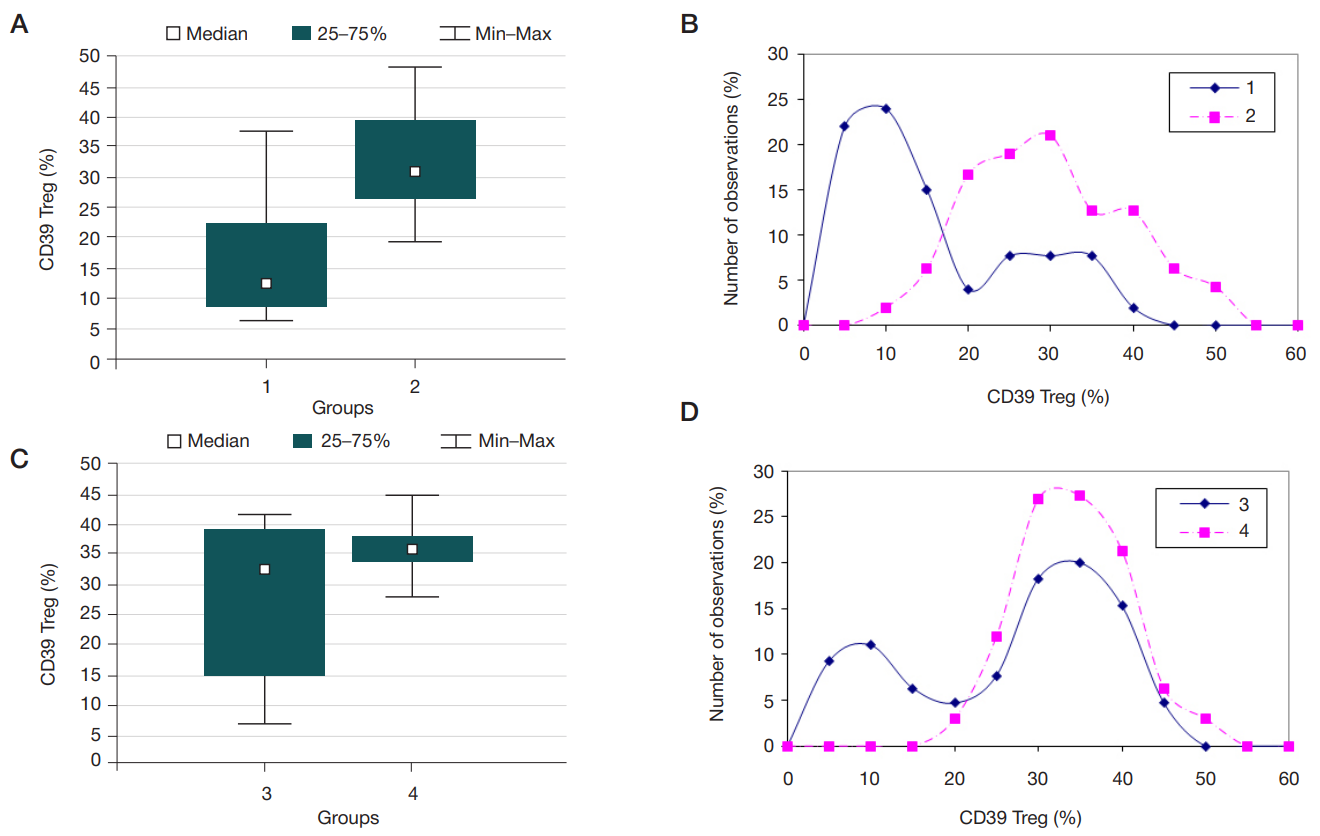
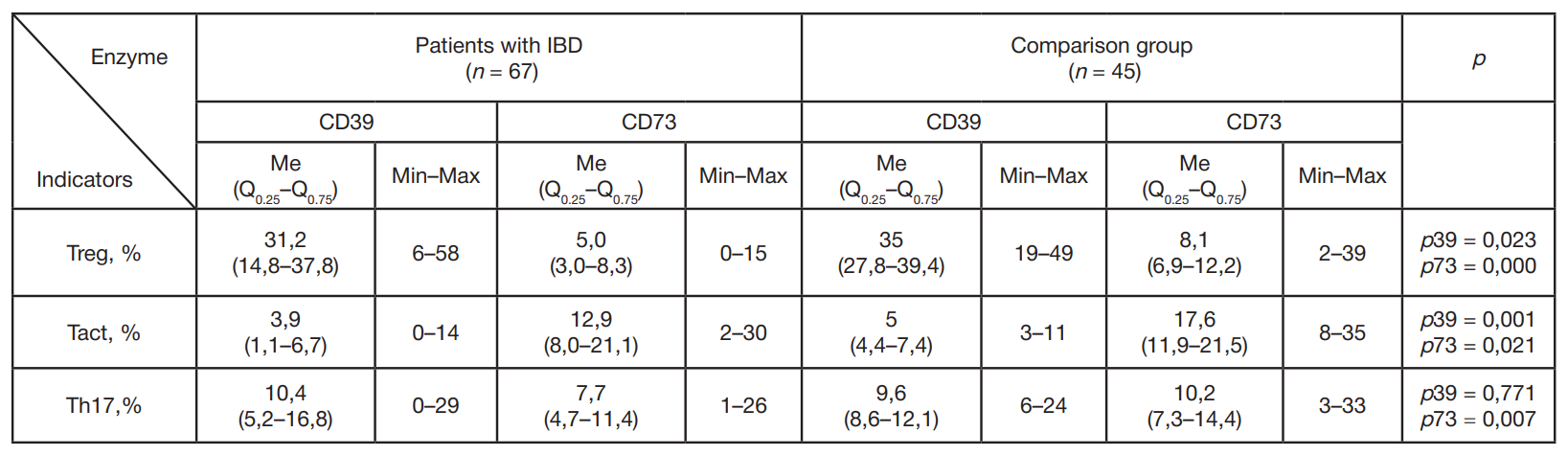
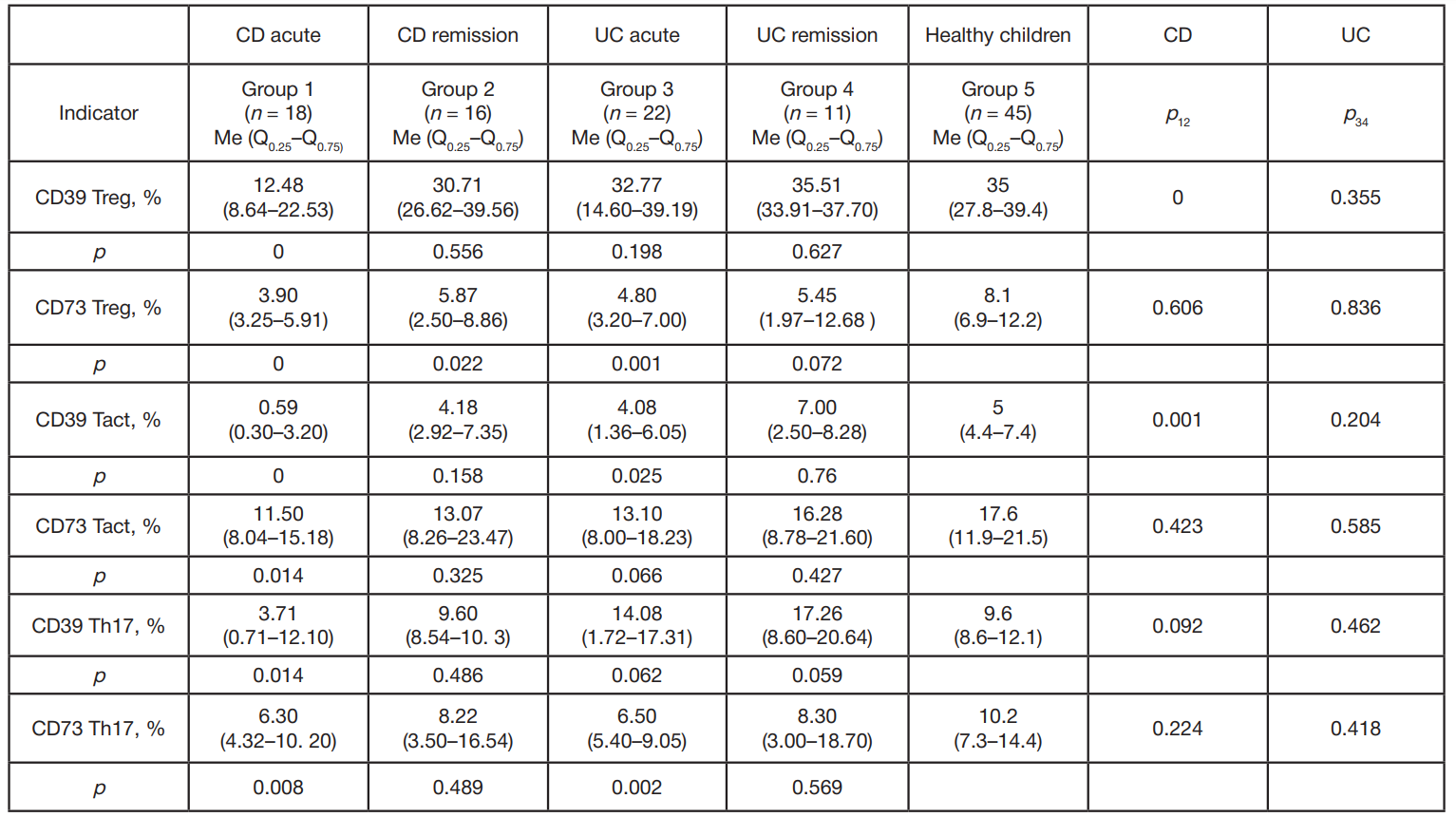
Content of CD4+ cells expressing CD39/CD73 ectonucleotidases in children with inflammatory bowel diseases
1 National Medical Research Center for Children's Health, Moscow, Russia
2 Sechenov First Moscow State Medical University, Moscow, Russia
Correspondence should be addressed: Tatiana V. Radygina
Lomonosovsky prospekt, 2/1, Moscow, 119296, Russia; ur.liam@anigidarvt
Funding: the study was carried out on state assignment of the Ministry of Health of Russia, number АААА-А19-119013090093-2.
Acknowledgement: the authors thank all patients for participation and acknowledge the Gastroenterology Department with Hepatology Group of the National Medical Research Center for Children's Health and its collaboration.
Compliance with ethical standards: the study was approved by the Ethics Committee of the National Medical Research Center for Children's Health, Moscow (protocol № 6 dated June 11, 2019). The informed consent was submitted by all study participants.