This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (CC BY).
ORIGINAL RESEARCH
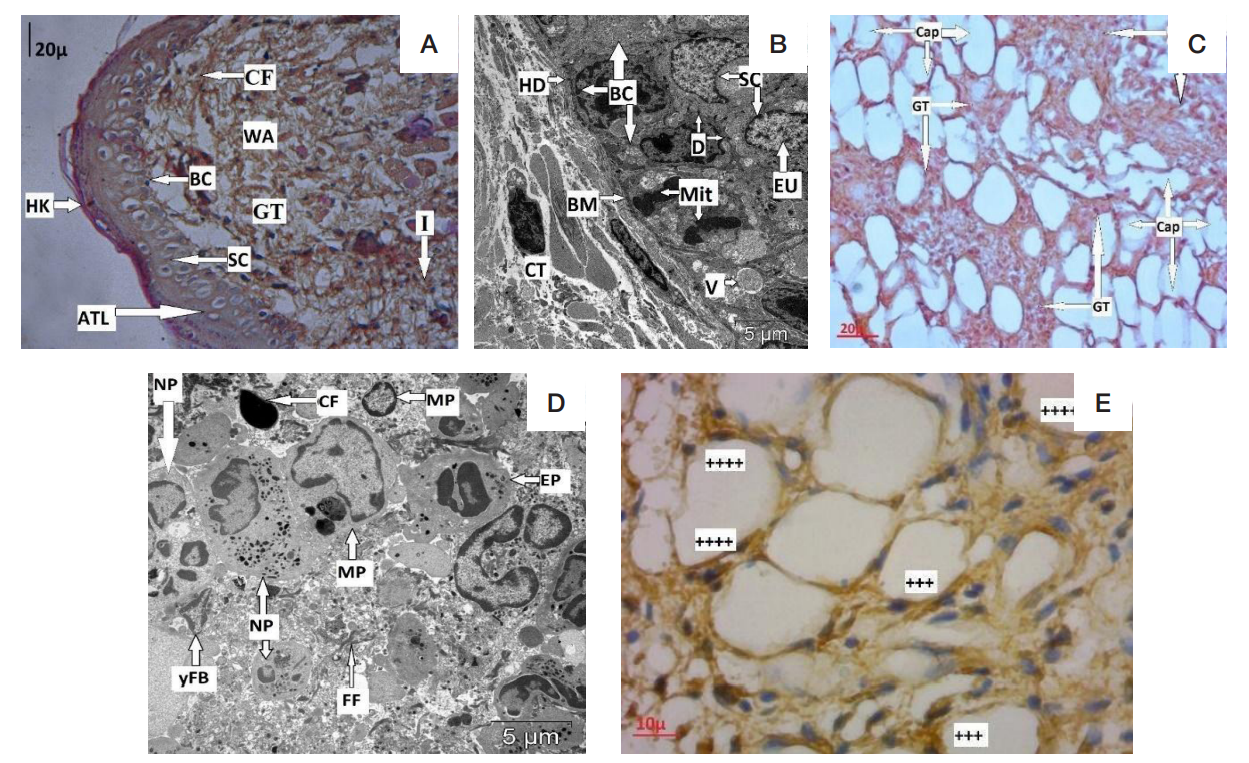
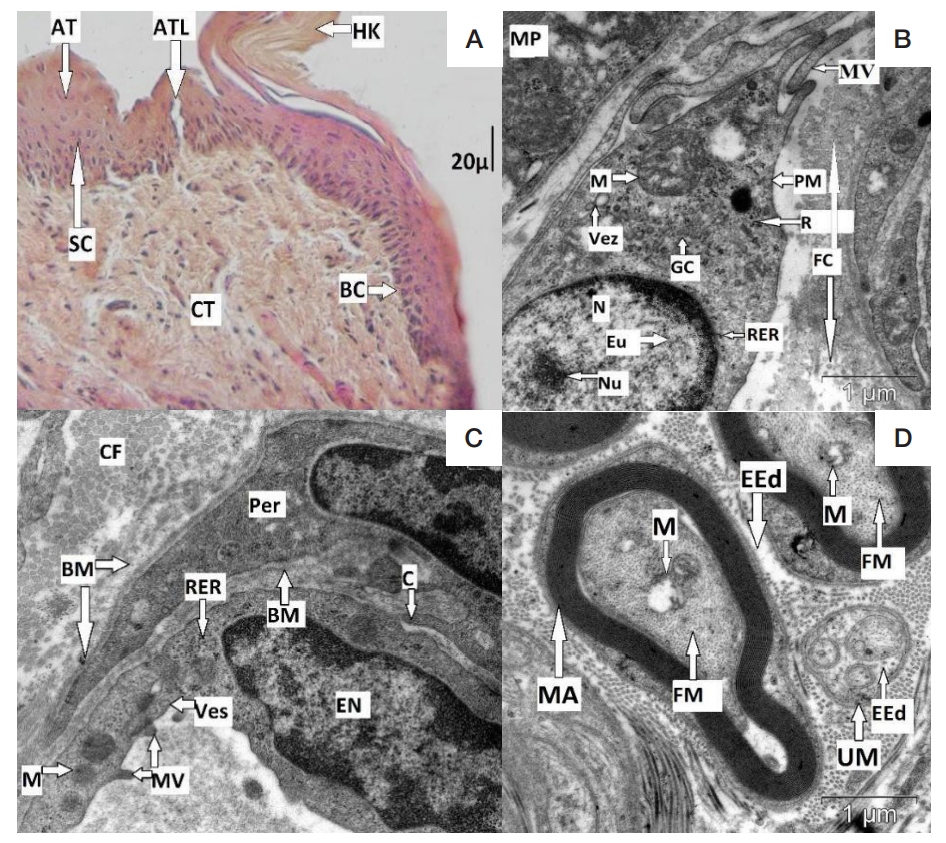
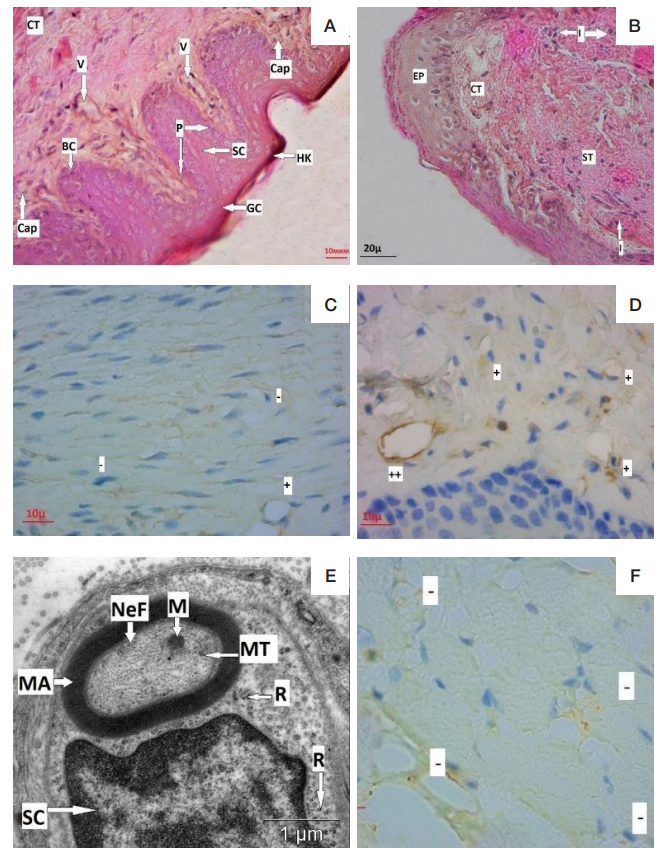
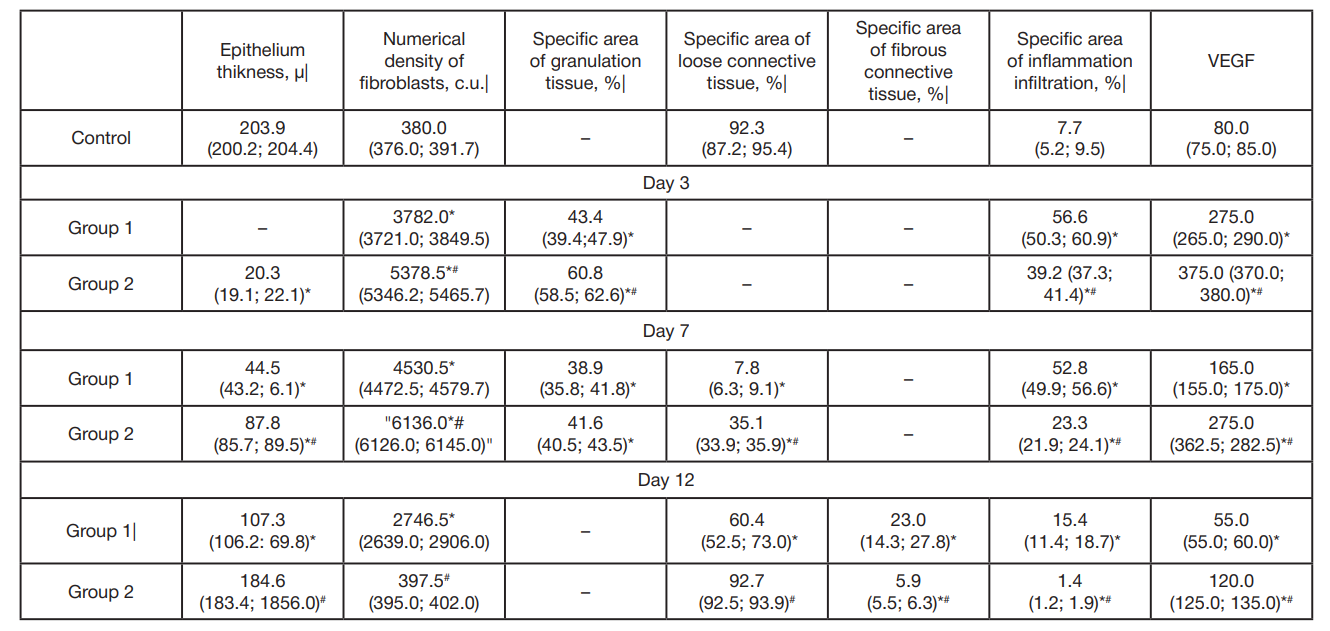
Morphological peculiarities of regeneration of oral mucosa associated with use of polymeric piezoelectric membranes
1 Siberian State Medical University, Tomsk, Russia
2 National Research Tomsk Polytechnic University, Tomsk, Russia
3 Montana State University, Bozeman, MT, USA
Correspondence should be addressed: Anastasiia D. Koniaeva
Moskovsky Trakt, 2, Tomsk, 634034, Russia; moc.liamg@59aynokaysa
Funding: the study was supported by the Russian Foundation for Basic Research under research project №23-25-00346.
Author contribution: Koniaeva AD, Varakuta EYu, Bolbasov EN, Stankevich KS — study concept and design; Koniaeva AD, Leiman AE — collection and processing of the material; Koniaeva AD, Varakuta EYu, Rafiev DO — text authoring; Koniaeva AD, Varakuta EYu, Rafiev DO, Bolbasov EN, and Stankevich KS — text editing.
Compliance with ethical standards: the study was approved by the Ethics Committee of the Siberian State Medical University (Minutes № 7693/1 of August 26, 2019). All manipulations with the animals were done as prescribed by the Directive 2010/63/EU of the European Parliament of September 22, 2010 "On the protection of animals used for scientific purposes", and the Declaration of Helsinki.