This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (CC BY).
ORIGINAL RESEARCH
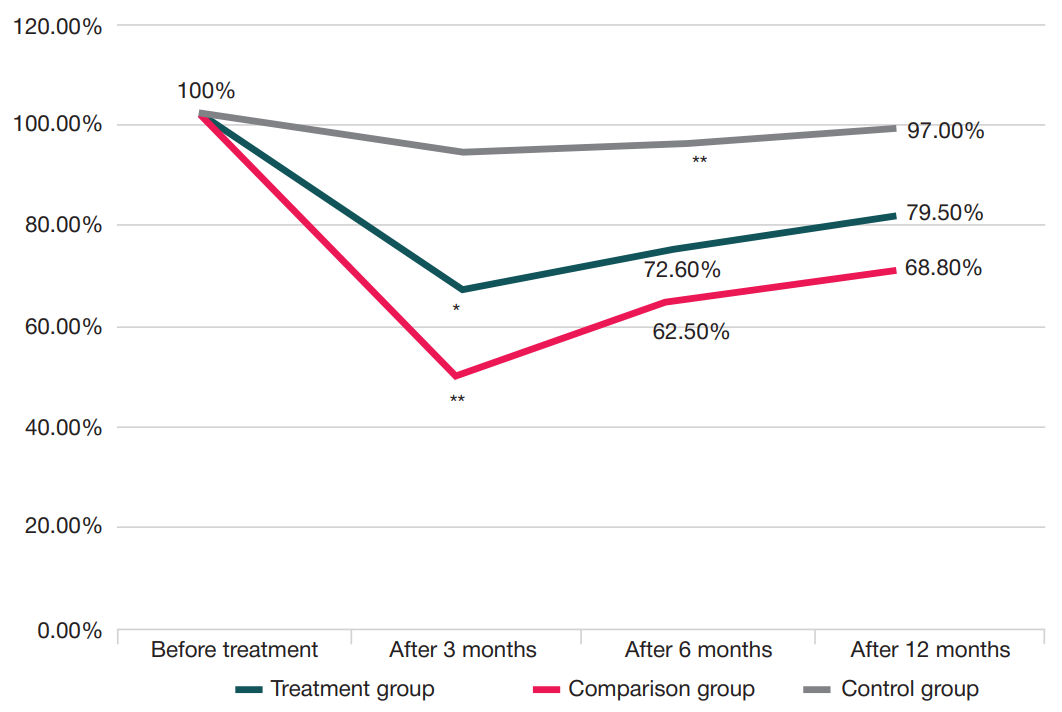
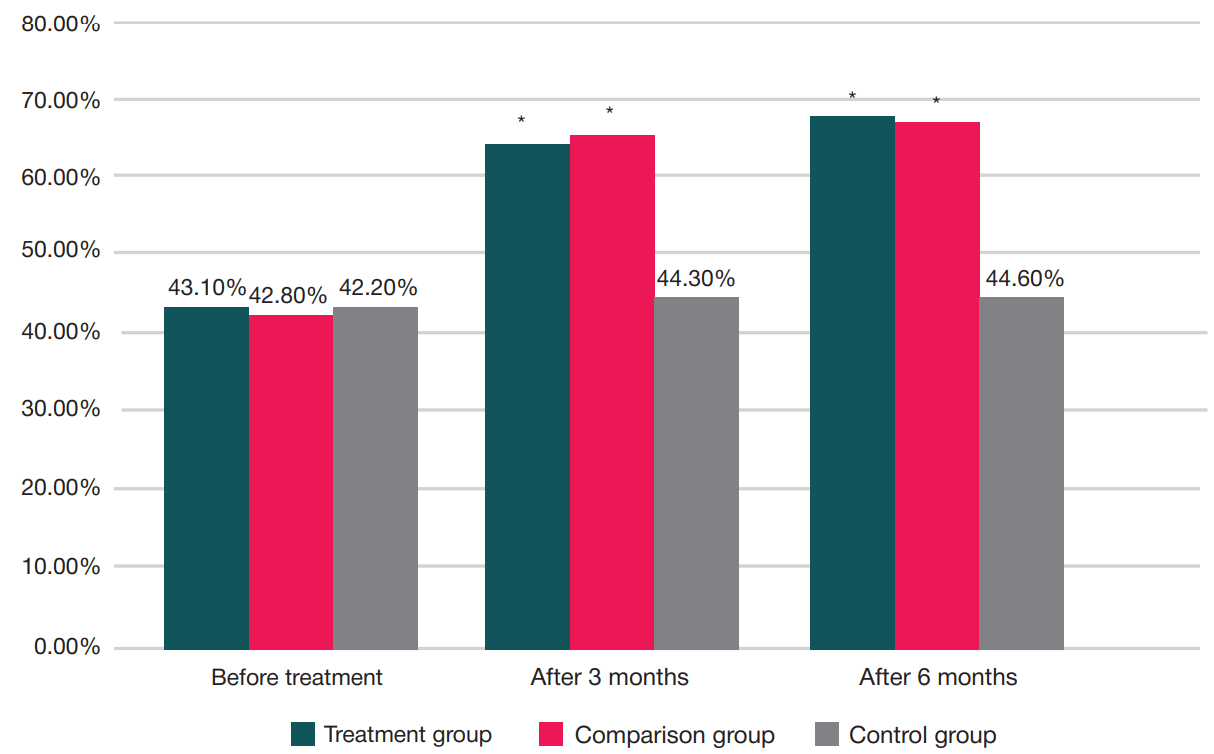
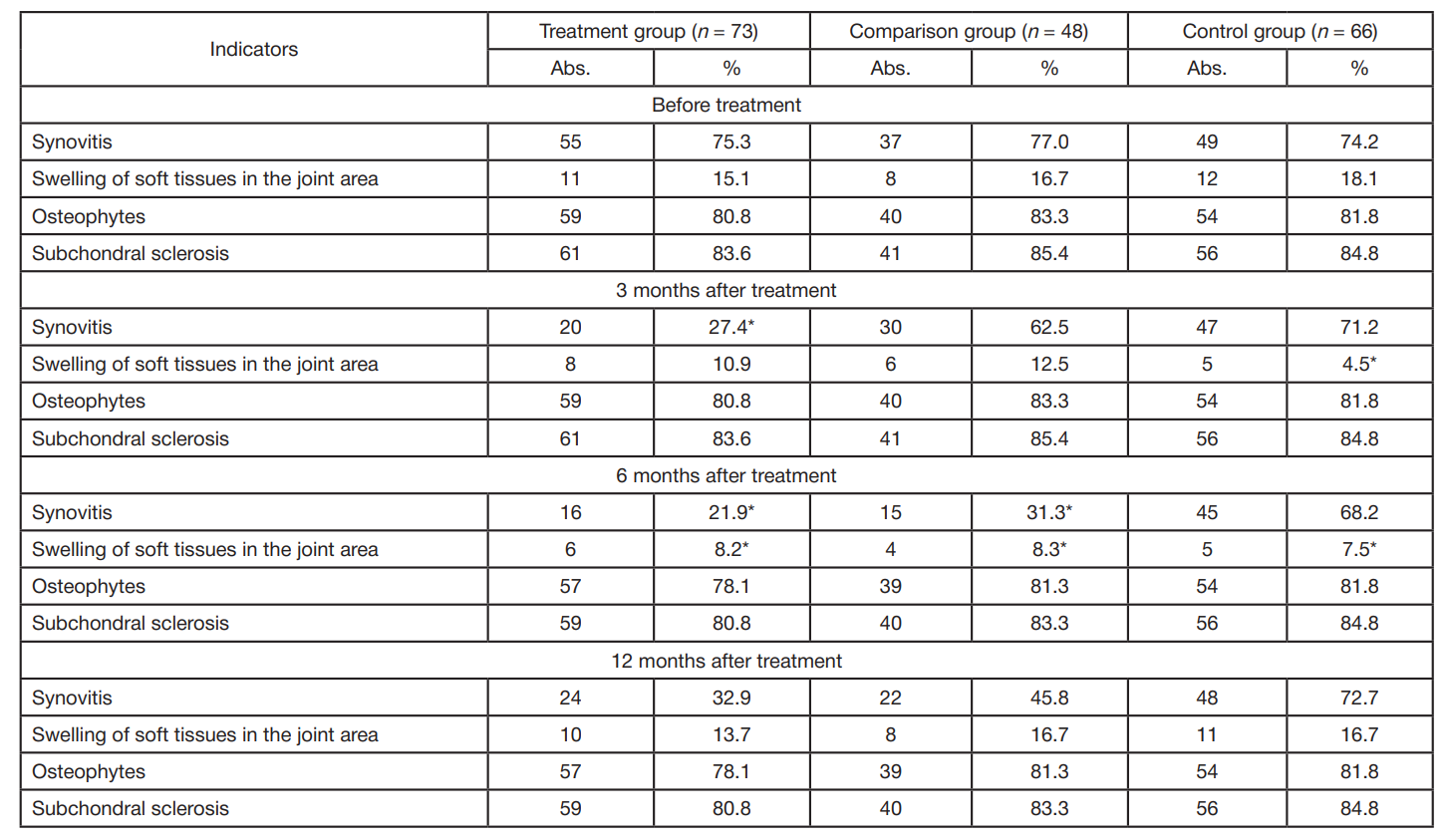
Treatment of gonarthrosis using autologous platelet-rich plasma
Pirogov Russian National Research Medical University, Moscow, Russia
Correspondence should be addressed: Maxim A. Danilov
Ostrovityanova, 1, Moscow, 117997, Russia; moc.liamg@volinad.dm
Author contribution: Egiazaryan KA, Danilov MA — study design development, analysis of results; Danilov MA, Abdusalamov RM — data collection, literature review, preparation administration, assessment of the results.
Compliance with ethical standards: the study was approved by the Ethics Committee of the N.I. Pirogov Russian National Research Medical University (Minutes #213 of December 13, 2021).