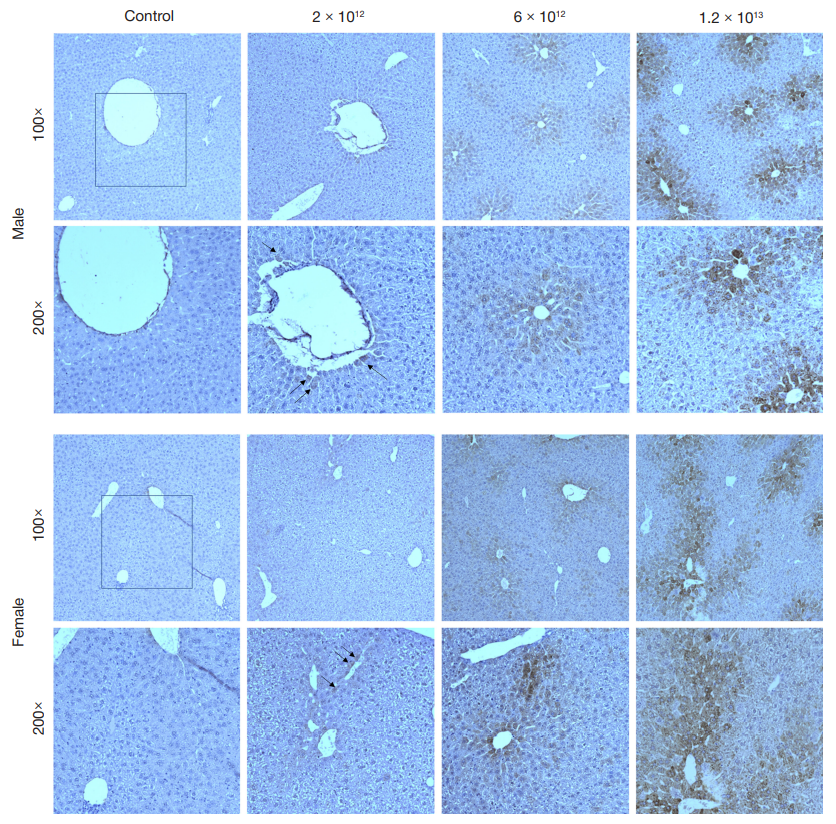
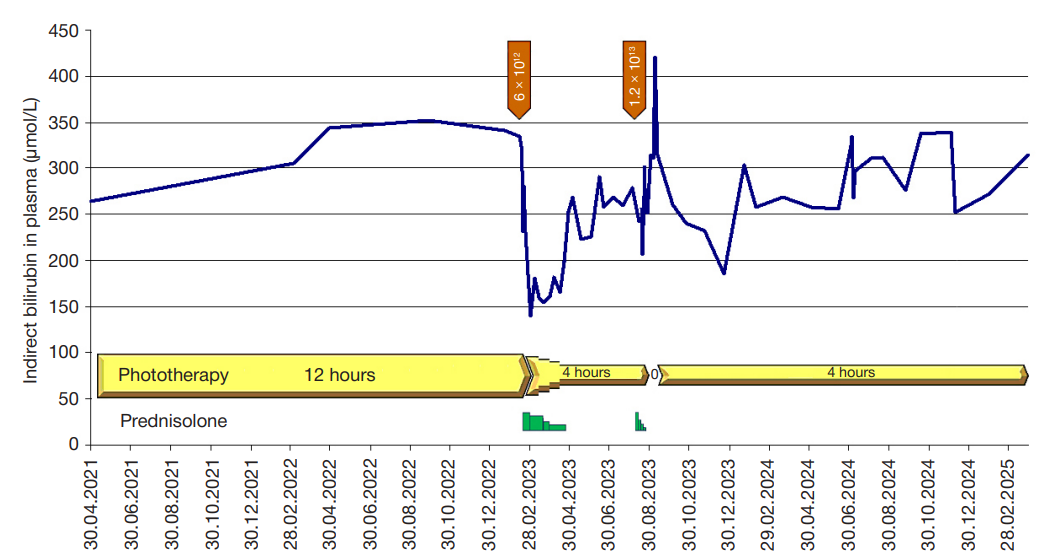
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (CC BY).
CLINICAL CASE
Two-step AAV8 gene delivery in a child with Crigler-Najjar syndrome type I
1 Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Moscow, Russia
2 Pirogov Russian National Research Medical University (Pirogov University), Moscow, Russia
Correspondence should be addressed: Denis V. Rebrikov
Ostrovityanova, 1, Moscow, 117997, Russia; moc.liamg@vokirberd
Funding: This work was supported by the Ministry of Health of the Russian Federation (No. 124020400004-9).
Author contribution: Rebrikov DV — literature analysis, study planning, development of study concept and design, development of drug concept, data interpretation, manuscript preparation; Degtyareva AV — literature analysis, study planning, development of study concept and design, patient examination, data interpretation; Gautier MS, Ushakova LV, Filippova EA — patient examination; Yanushevich YuG, Gorodnicheva TV, Bavykin AS — drug development, data interpretation, manuscript preparation; Degtyarev DN, Sukhikh GT — development of study concept and design, data interpretation.
Compliance with ethical standards: the therapy was approved by Ethics Committee at the Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology on Dec 01 2022, Protocol #12 for infusion I, and on Jul 20 2023, Protocol #7 for infusion II. The patient's legal representatives provided voluntary informed consents for the study and for each infusion of the drug.