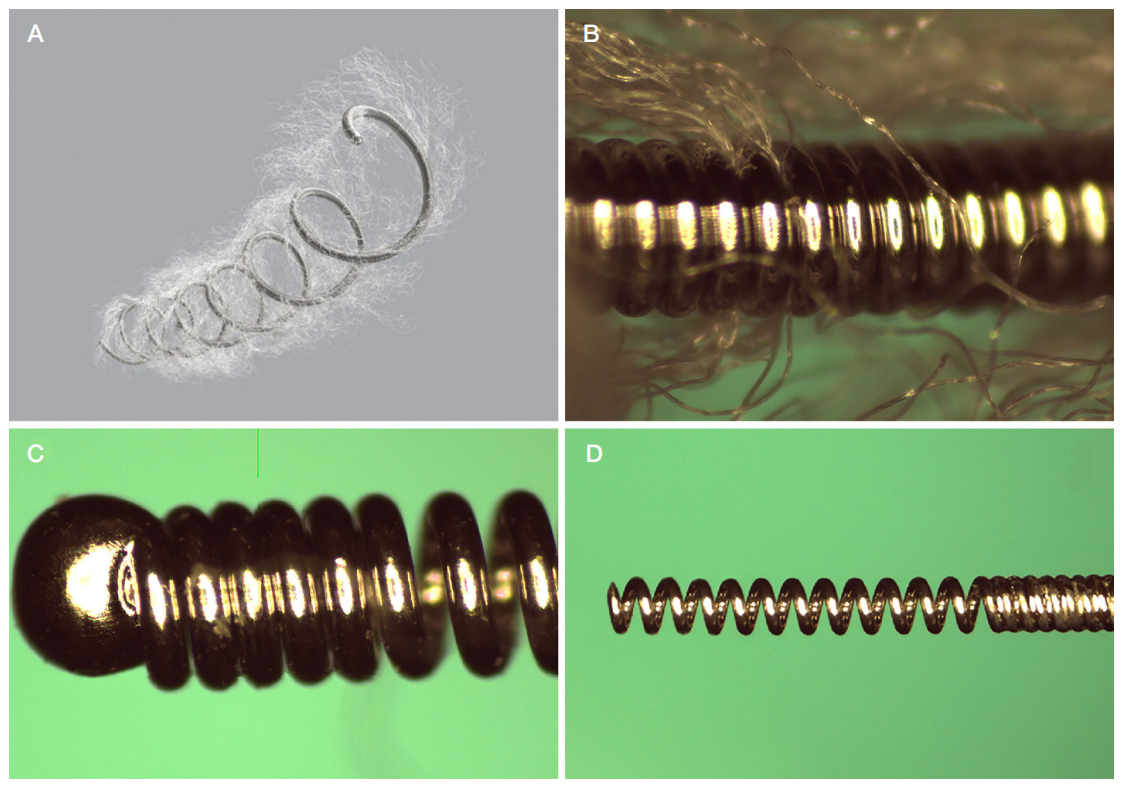
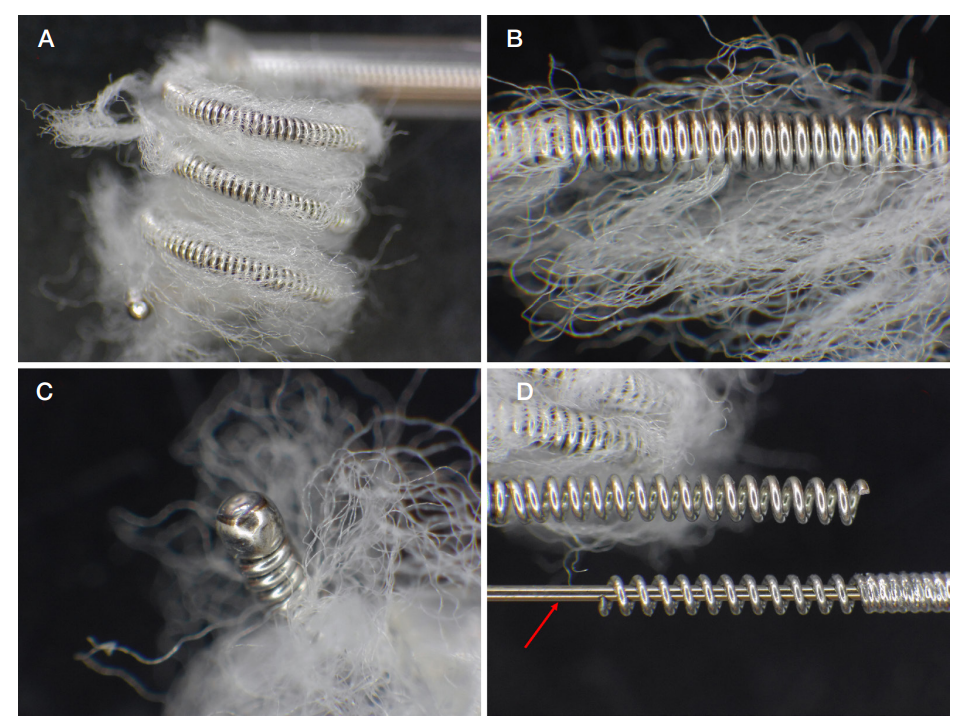
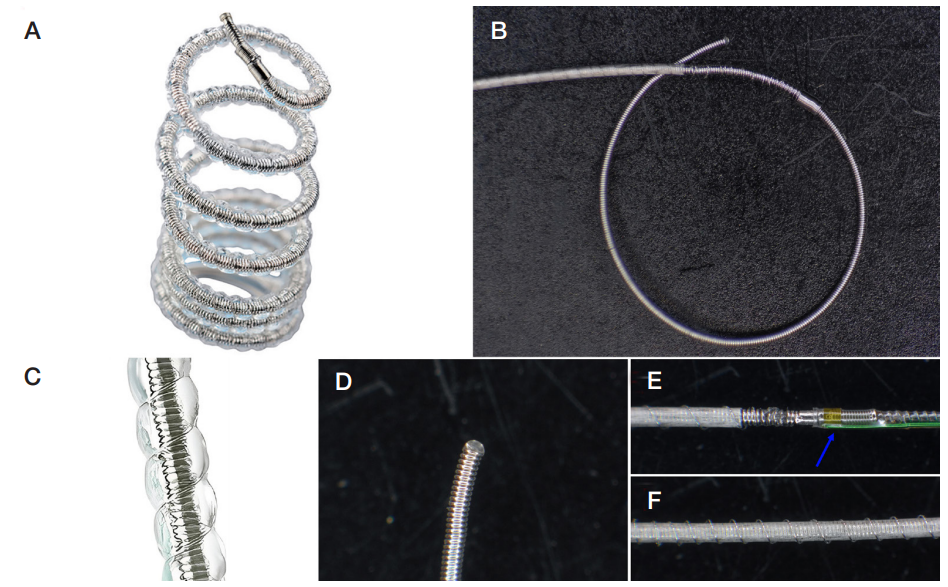
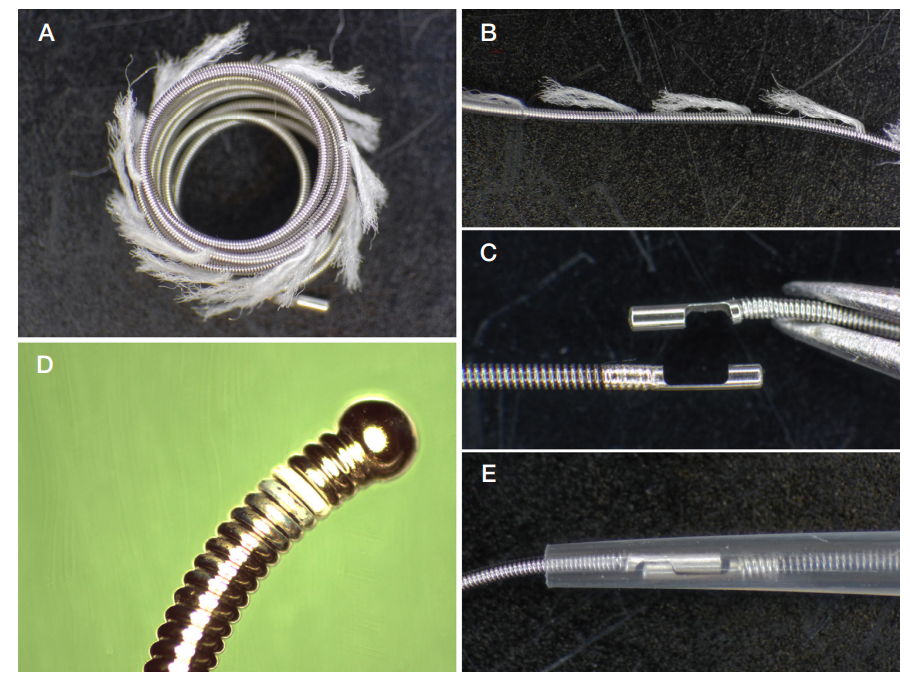
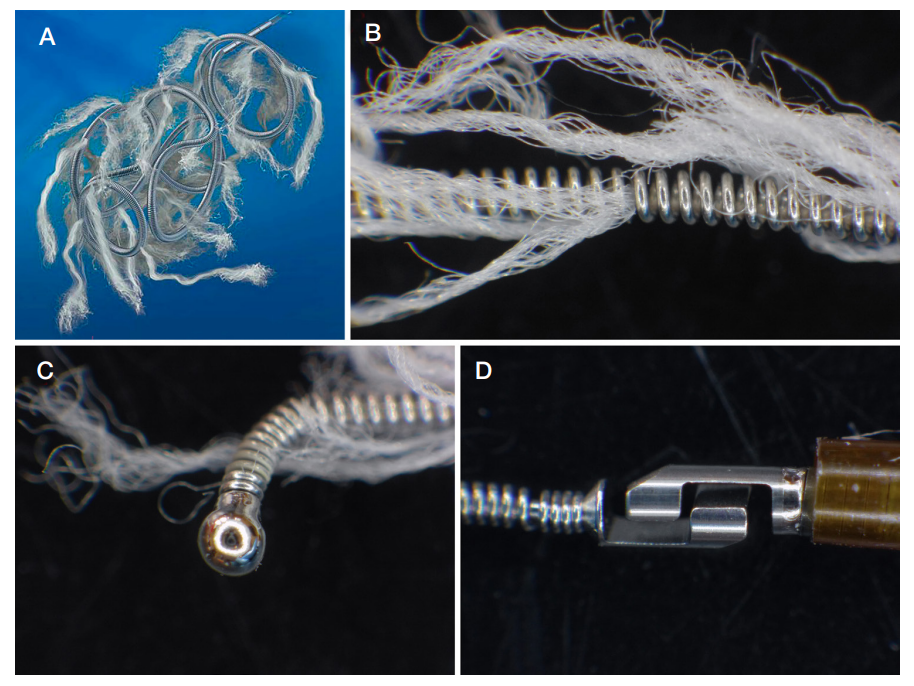
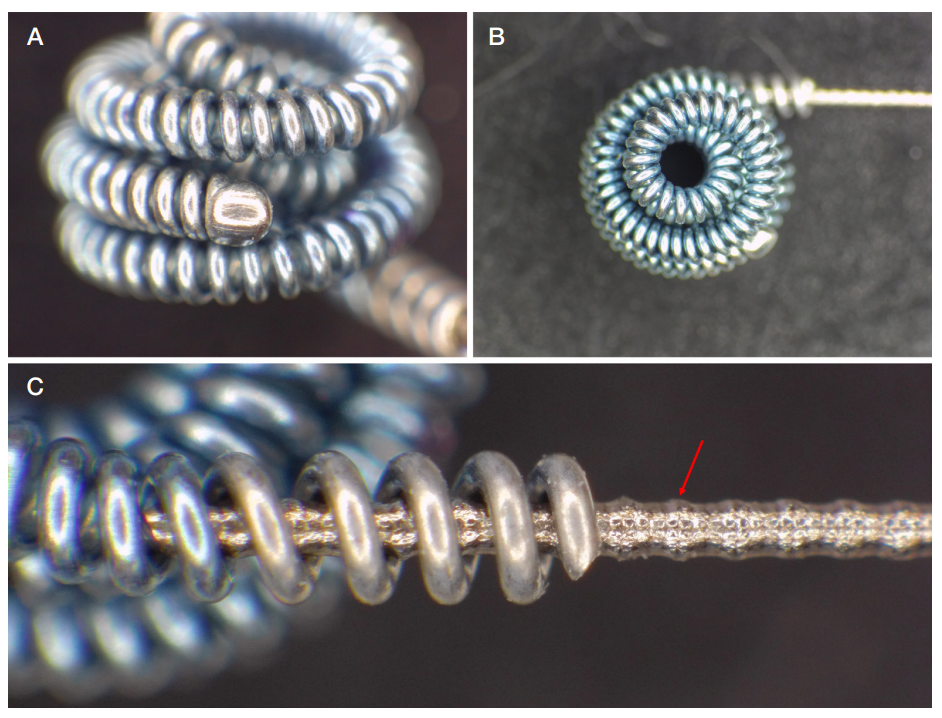
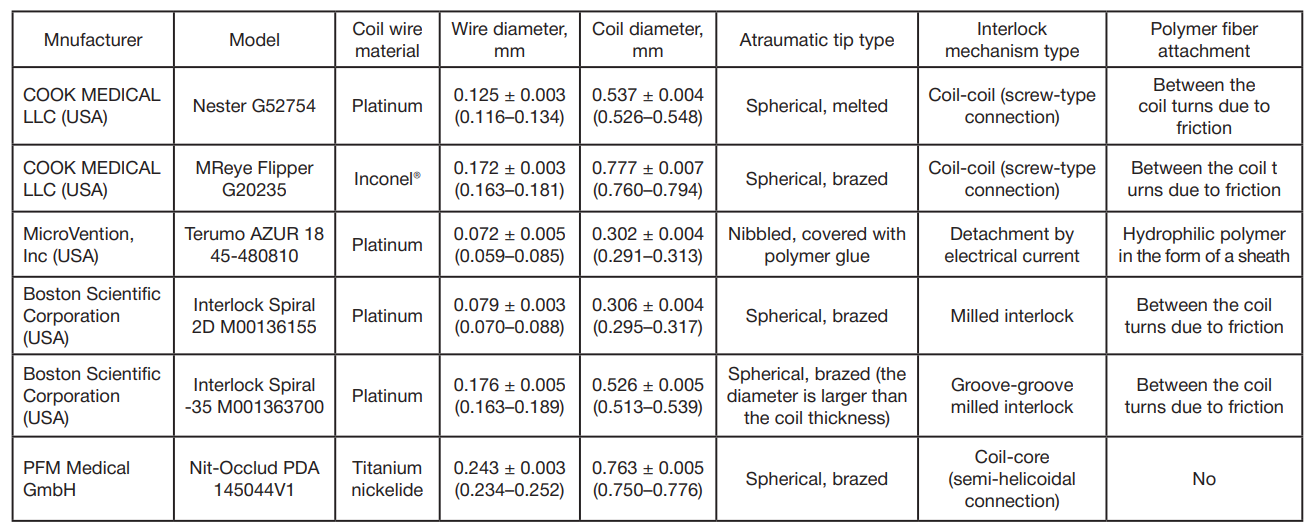
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (CC BY).
ORIGINAL RESEARCH
Comparative analysis of metallic endovascular coil frame designs
1 Meshalkin National Medical Research Center, Novosibirsk, Russia
2 Novosibirsk State Medical University, Novosibirsk, Russia
Correspondence should be addressed: Elena V. Chepeleva
Rechkunovskaya, 15, Novosibirsk, 630055, Russia; ur.niklahsem@avelepehc_e
Funding: the study was conducted as part of the Russian Science Foundation project No. 25-15-00480.
Author contribution: Chepeleva EV — data analysis, manuscript writing and editing; Kozyr KV, Borodin VP — experimental procedure, data analysis, visualization of findings; Khakhalkin VV — study concept and design, data analysis; Vladimirov SV — experimental procedure, data analysis, visualization of findings; Makhmudov MA, Badoian AG, Baranov AA — technical support, data validation, graphics design; Krestyaninov OV — general research management, coordinating work, editing and approval of the final version of the manuscript.