This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (CC BY).
ORIGINAL RESEARCH
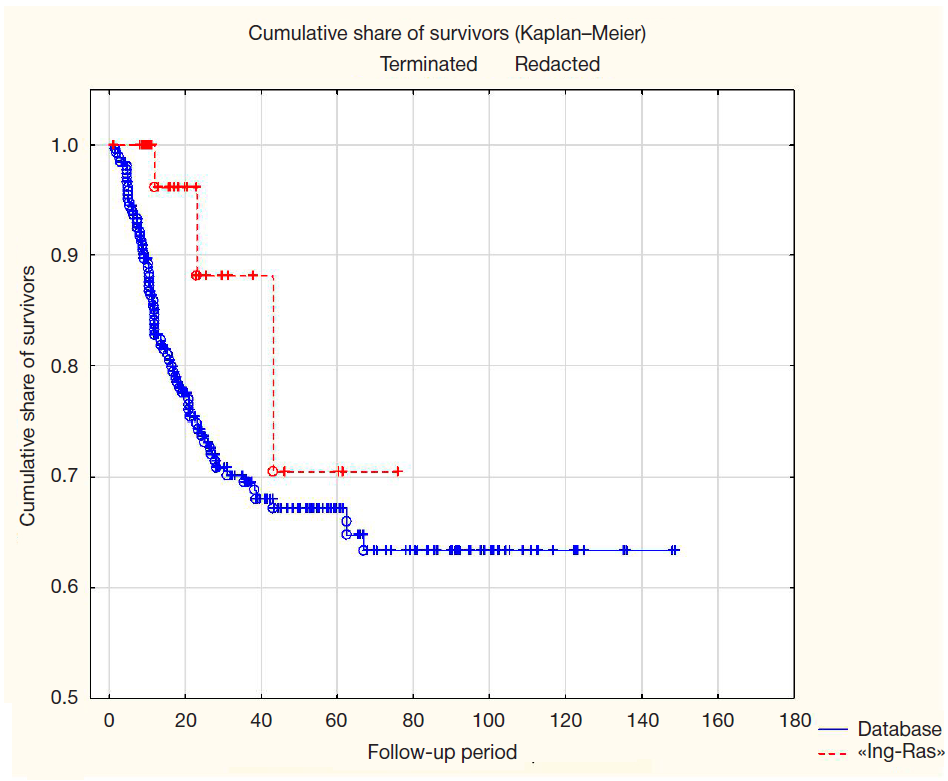
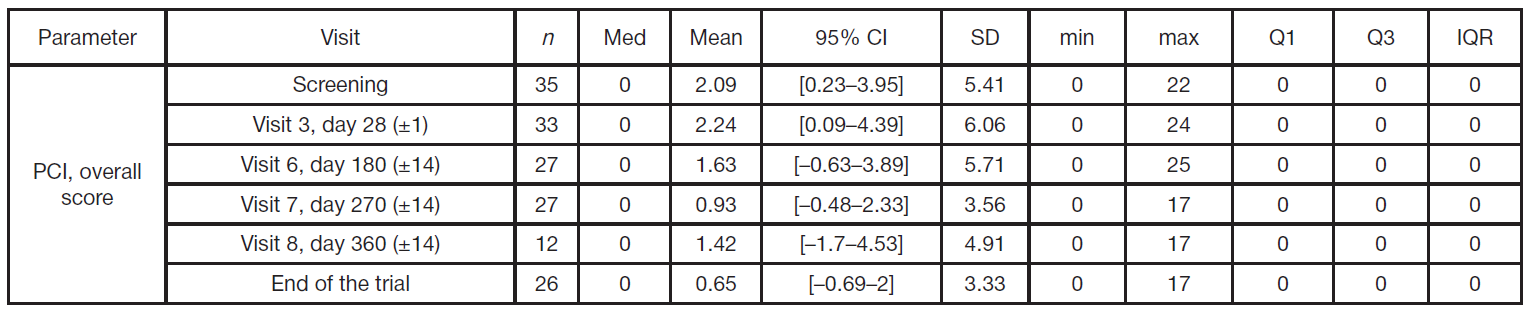
Results of a Phase IIa clinical trial of a RAS-GTPase inhibitor (Ing-Ras) for the treatment of gastrointestinal tumors
Russian Scientific Center for Roentgenoradiology, Moscow, Russia
Correspondence should be addressed: Ilya A. Puchkov
Profsoyuznaya, 86, 117997, Moscow, Russia; ur.liam@vokhctuop
Funding: the study was conducted with financial support from the Ministry of Health of the Russian Federation, the State Task project EGISU No. 1023021500033- 4-3.2.21;3.1.5.
Author contribution: Kulinich TM — research concept and design, material collection and processing, data analysis, manuscript writing; Kukoleva EA — formation of patient groups, a set of clinical material, analysis of results, manuscript writing; Goncharov SV — design of the experimental part of the study, formation of patient groups, a set of clinical material, analysis of results; Kudinova EA, Goncharova OI — analysis of the clinical and experimental research results, manuscript writing and editing; Puchkov IA, Kaminsky VV — formation of patient groups, analysis of results, manuscript writing and editing; Bozhenko VK — research concept and design, manuscript editing and approval.
Compliance with ethical standards: the study was approved by the Ethics Committee of the Russian Scientific Center of Roentgenoradiology (protocol No. 4 dated 28 April 2023). The 2022-1-“Инг-Рас” trial was approved by the Ministry of Health of the Russian Federation (approval No. 177 for the clinical trial dated 30.03.2023). It was conducted in accordance with the ethical principles set out in the World Medical Association Declaration of Helsinki.