This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (CC BY).
ORIGINAL RESEARCH
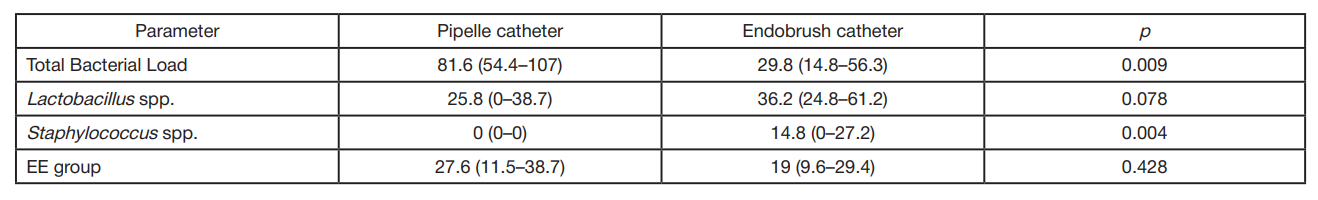
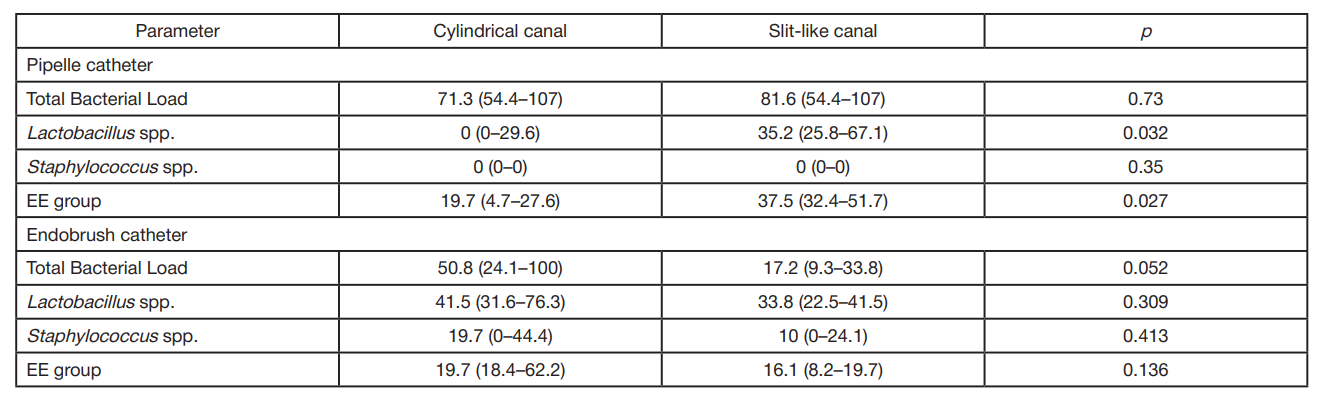
Pipelle and endobrush catheters do not prevent contamination of endometrial samples by cervical microbiota
1 Ural State Medical University, Yekaterinburg, Russia
2 Medical Center “Garmonia”, Yekaterinburg, Russia
3 LLC “Quality Med”, Yekaterinburg, Russia
Correspondence should be addressed: Danila L. Zornikov
Kluchevskaya, 17, Yekaterinburg, Russia, 620109; moc.liamg@ldvokinroz
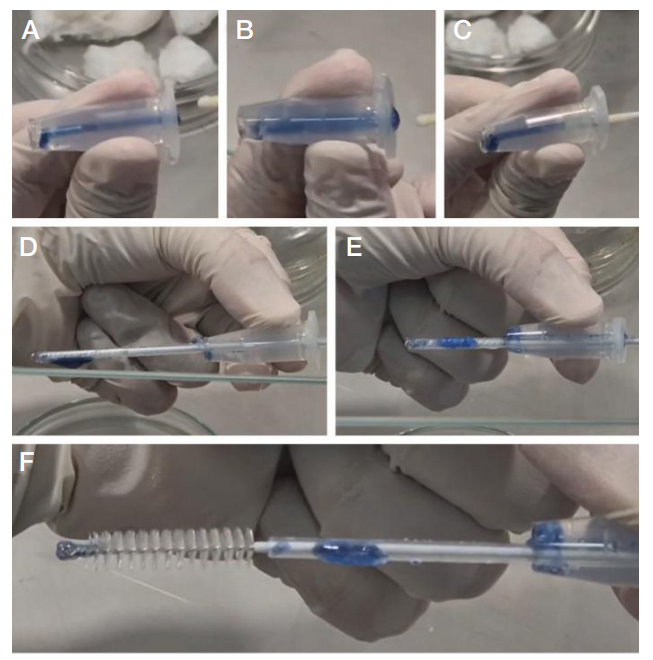
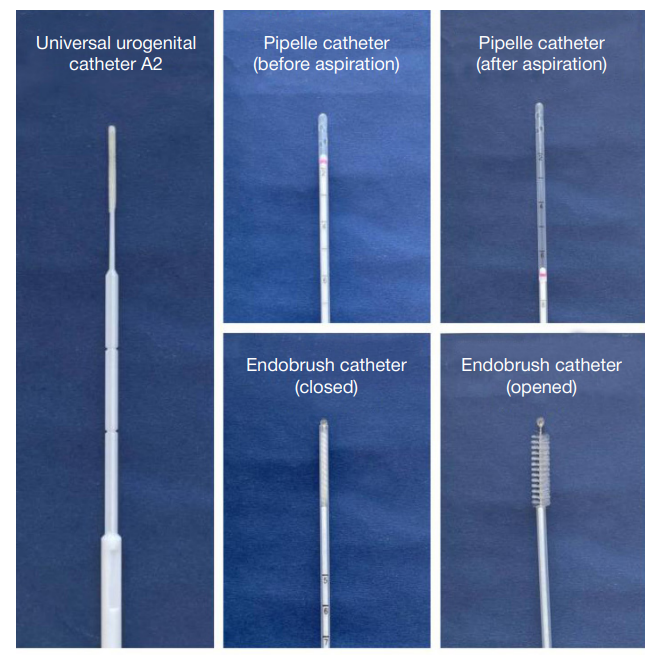
Author contribution: Zornikov DL — study organization, data analysis, statistical analysis, writing and editing of the article; Bekhter AA — development and creation of in vitro cervical models; Kornilov DO — development and creation of in vitro cervical models, preparation of illustrations; Simarzina VM — execution of experimental work (sampling from models), manuscript preparation; Nechaeva DM — execution of experimental work (sampling from models), performance of molecular genetic studies (PCR), manuscript preparation; Karyakina AE — performance of molecular genetic studies (PCR), preparation of the manuscript and illustrations; Leshukova MA — manuscript preparation; Amineva PG — preparation and provision of bacterial cultures for analysis; Voroshilina ES — scientific supervision and coordination, analysis and interpretation of data, writing and editing of the article.
Compliance with ethical standards: the study was approved by the Ethics Committee of Ural State Medical University (Protocol № 3 dated June 20, 2025).