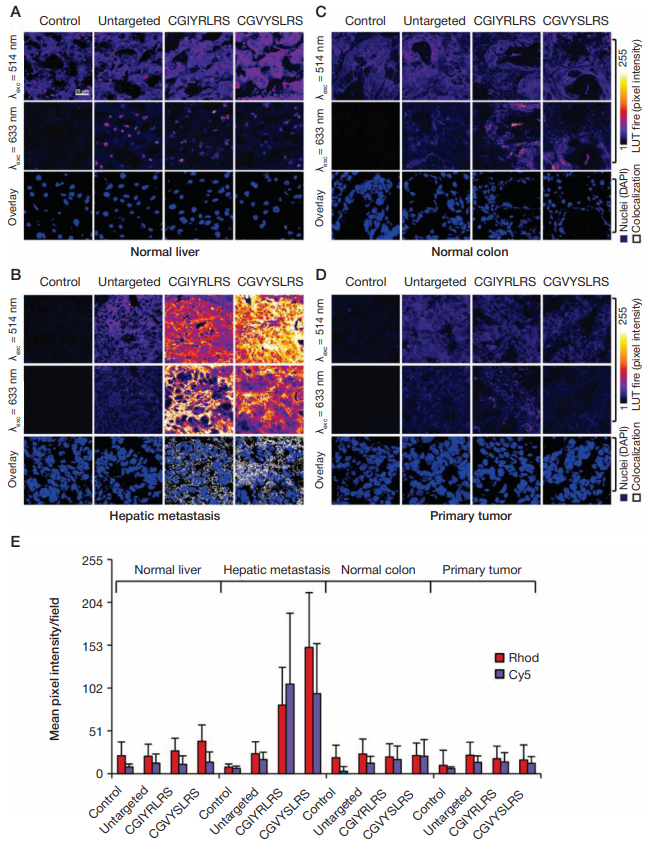
Fig. 1. Frozen sections of (A) normal liver, (B) hepatic metastasis, (C) normal colon and (D) primary tumor were fixed in 4% formaldehyde, before incubation with untargeted, CGIYRLRS-, or CGVYSLRS-(Rhod+Cy5)-SPNs for 4 hours at room temperature. After washing, SPN-emitted fluorescence was analyzed by confocal microscopy and the output was converted into the false-color LUT Fire scale for prompt visualization. Nuclei were stained with 4',6-diamidino-2-phenylindole (DAPI).
Colocalized pixels were identified by ImageJ software. Experiments were performed with similar results on specimens from 10 patients with metastatic CRC; exemplary
images from tissues of patient #P85 are shown. (E) Quantification of SPN binding is expressed as the intensity of emitted pixel following excitation at 514 nm (Rhod)
and 633 nm (Cy5), and represents a mean value of 5 images for each tissue. From Soster et al. [5]
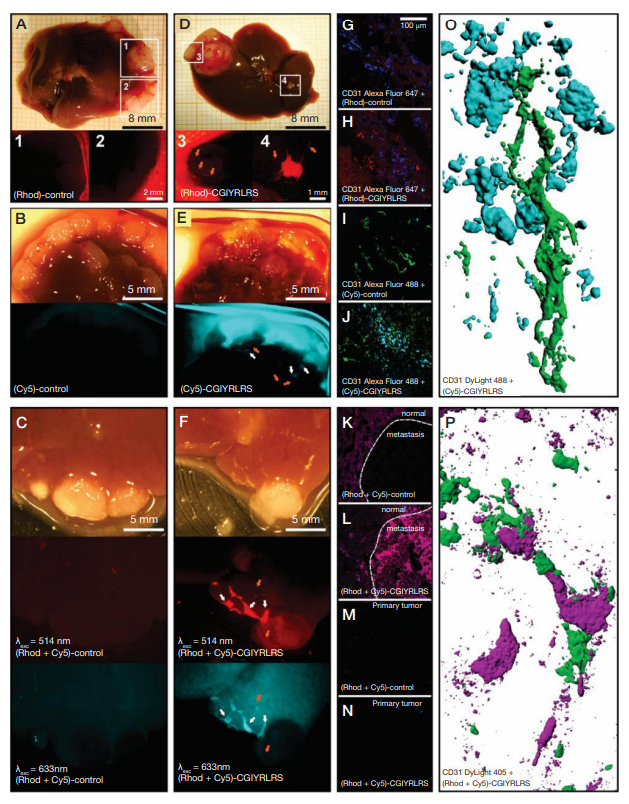
Fig. 2. NOD/SCID mice bearing a primary tumor and multiple liver metastases were injected with single- [control (A, Rhod; B, Cy5) or CGIYRLRS- (D, Rhod; E, Cy5)] or dual-color [control (C) or CGIYRLRS (F)] SPNs. After 16 hours, mice were euthanized and explanted organs were photographed with a high-resolution digital camera connected to a fluorescence stereomicroscope. In D, E, and F, orange arrows indicate blood vessels crossing the hepatic metastasis; in E and F, white arrows indicate sub-millimetric metastatic foci. Samples of the same tissues were OCT-frozen, cut into 10-μm slices, and evaluated by confocal analysis of single- [control (G, Rhod; I, Cy5), CGIYRLRS (H, Rhod; J, Cy5)] or dual-color [control (K), CGIYRLRS (L)] fluorescence. To visualize blood vessels, staining for CD31 was superimposed to the SPNs signal and visualized by the secondary antibodies Alexa Fluor®647 (G–H), Alexa Fluor®488 (I–J) and DyLightTM405 (K–N), for overlay with (Rhod)-SPNs, (Cy5)-SPNs and dual-color SPNs, respectively. In the case of dual-color SPNs, samples of primary tumors from mice injected with either control (M) or CGIYRLRS (N) SPNs are visualized as a further negative control. In O (detail of the field in J) and P (detail of the field in L), tridimensional models of 50–80 confocal image series were reconstructed with IMARIS software. From Soster et al. [5]
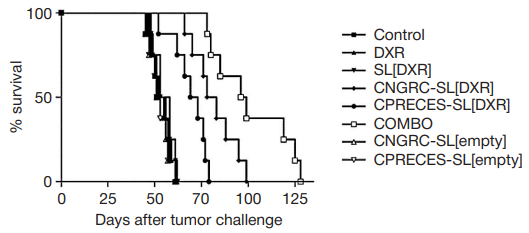
Fig. 3. Therapeutic efficacy of APN- and APA-targeted liposomal formulations in mouse models of neuroblastoma. Nude mice (8/group) implanted orthotopically with
human neuroblastoma cells were treated (starting 21 days after tumor implant) by intravenous administration of HEPES-buffered saline (control), CNGRC-SL[empty],
CPRECES-SL[empty] or 5 mg/kg of DXR, either free (DXR) or encapsulated in untargeted (SL[DXR]), APN- (CNGRC-SL[DXR]) or APA-targeted (CNGRC-SL[DXR])
liposomes or an equimolar mixture of CNGRC-SL[DXR] and CNGRC-SL[DXR] (COMBO), once-a-week for a total of 5 weeks. The efficacy of each formulation was
evaluated in terms of survival and is expressed in a Kaplan–Maier graph as % of alive mice at different timepoints. From Loi et al. [18]
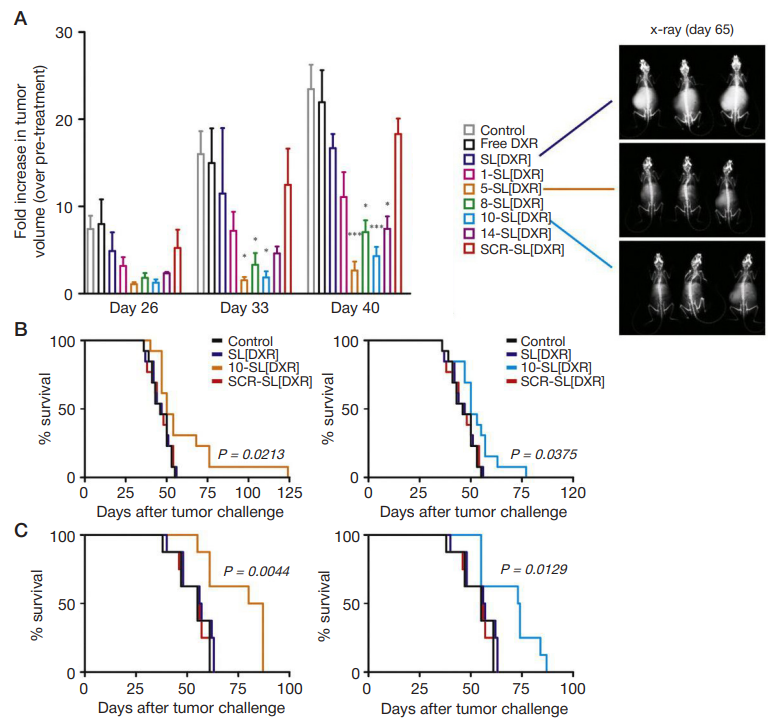
Fig. 4. (A) Therapeutic efficacy of peptide-targeted liposomal formulations in mouse models obtained by orthotopic implant of luciferase-expressing human neuroblastoma cells. Treatments started 21 days after tumor implant. Mice (5/group) were administered intravenous with HEPES-buffered saline (control), or 5 mg/kg of DXR, either free (DXR) or encapsulated in untargeted (SL[DXR]), scramble peptide- (SCR-SL[DXR] or targeting peptide-functionalized (1-, 5-, 8-, 10-, 14-SL[DRX])
liposomes, once-a-week for a total of 3 weeks. Tumor growth was monitored by BLI 5 days after each treatment (days 26, 33, 40 from tumor implant). Values are
reported as fold increase in tumor volume compared to pre-treatment (day 20). Exemplary pictures of X-ray acquisitions one month after the end of treatment (day 65),
relative to mice treated with SL[DXR], 5-SL[DXR] or 10-SL[DXR], are shown. (B–C) Therapeutic effect of the targeted formulations evaluated in terms of overall survival
in both the pseudo-metastatic model (13 animals/ group, B) and the orthotopic model (8 animals/group, C). Statistical analysis: A, p vs. SL[DXR]; B–C, p vs. control, SL[DXR] and SCR-SL[DXR]. From Loi et al. [16].
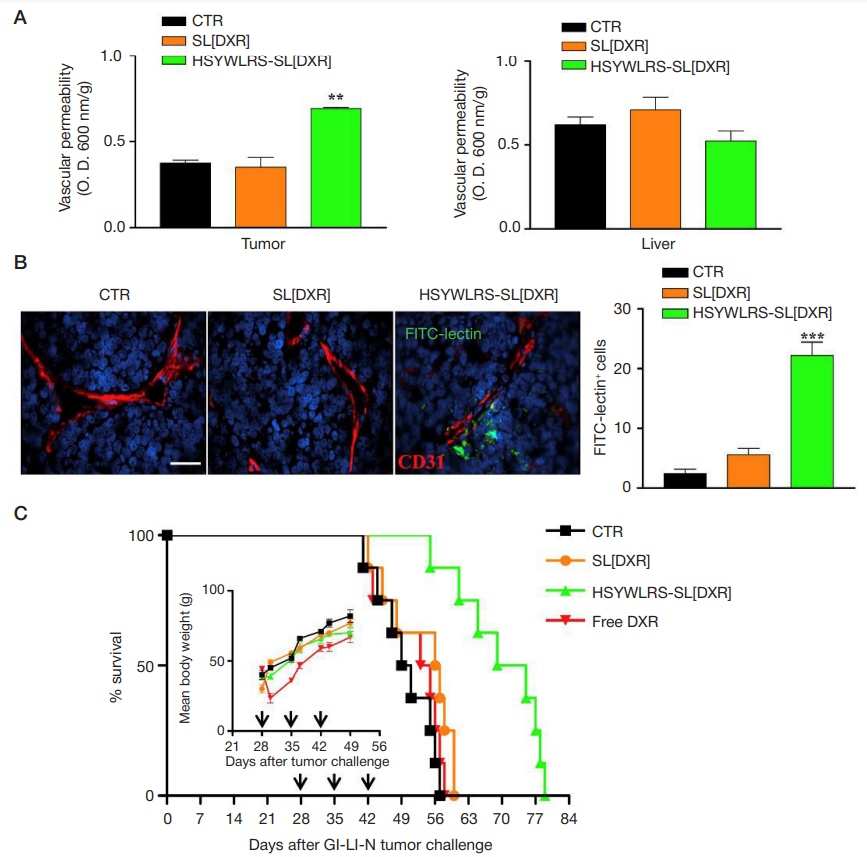
Fig. 5. (A) In vivo systemic permeability. Mice (3/group) bearing orthotopic tumors were treated, 28 days after, with a single bolus of DXR (5 mg/kg), encapsulated into untargeted (SL[DXR]) or HSYWLRS-targeted (HSYWLRS-SL[DXR]) liposomes, in combination with 1 mg of Evans Blue dye. Control mice (CTR) received HEPESbuffered saline only. One hour after, mice were perfused, tumors and livers collected and Evans Blue extracted and quantified (O.D. 600 nm). Results are expressed
as Evans Blue dye per g of tissue. * *, p < 0.01: HSYWLRS-SL[DXR] vs. CTR and SL[DXR]. (B) Exemplary tumor sections from control mice or from mice treated with SL[DXR] or HSYWLRS-SL[DXR] and inoculated with FITC-lectin (green). Red: CD31. Blue: cell nuclei (DAPI). Scale bar: 40 μm. Graph on the right, numbers of FITClectin positive cells. * * *, p < 0.001, HSYWLRS-SL[DXR] vs. CTR and SL[DXR]. (C) Potentiated therapeutic efficacy of HSYWLRS-SL[DXR]. Mice (8/group) bearing orthotopic tumors were treated intravenous with 5 mg/kg of DXR, either free (free DXR) or encapsulated into SL[DXR] or HSYWLRS-SL[DXR] liposomes, once-a-week for 3 weeks (arrows). Control mice received HEPES buffer only (CTR). Survival: p < 0.0008: HSYWLRS-SL[DXR] vs. SL[DXR]). Insert: mean body weight at different
timepoints. From Cossu et al. [17]
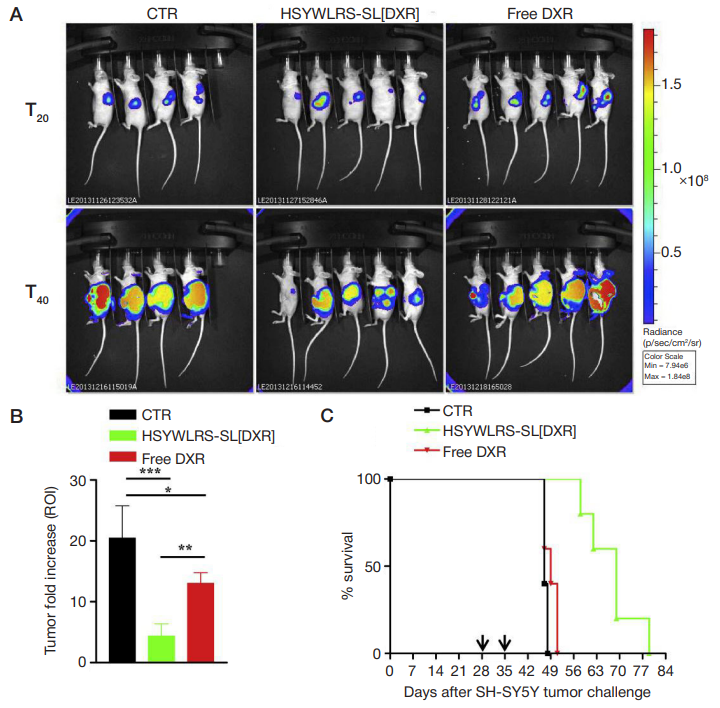
Fig. 6. (A) Lateral (tumor side) images from mice orthotopically implanted with luciferase-expressing human neuroblastoma cells. Animals were treated intravenously, once a week for 2 weeks (arrows), with 5 mg/kg of DXR, either free (free DXR) or encapsulated into HSYWLRS-targeted liposomes (HSYWLRS-SL[DXR]). CTR mice received HEPES-buffered saline. Tumor growth was monitored by BLI at day 20 (before treatment) and 40 (end of treatments) after tumor challenge. (B) Antitumor effects at the end of treatments; values are reported as fold increase in tumor volume at day 40 over day 20. *, p < 0.05: free DXR vs. CTR; * *, p < 0.01: HSYWLRSSL[DXR] vs. free DXR; * * *, p < 0.001: HSYWLRS-SL[DXR] vs. CTR; (C) HSYWLRS-SL[DXR] show potentiated therapeutic efficacy. Survival: p < 0.0025: HSYWLRSSL[DXR] vs. CTR and free DXR. From Cossu et al. [17]
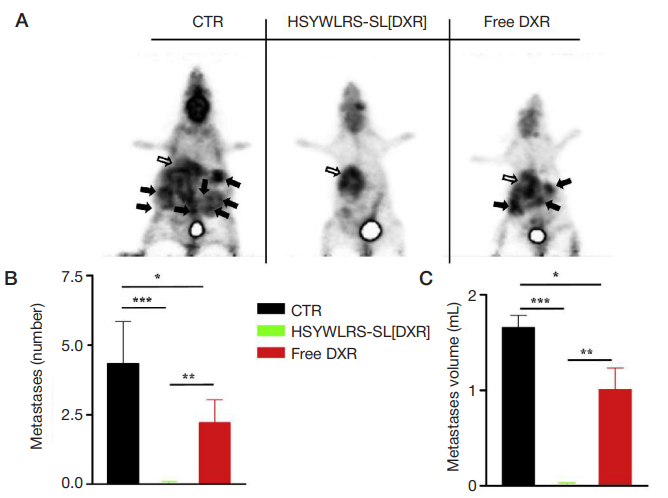
Fig. 7. Treatment with HSYWLRS-SL[DXR] inhibits secondary tumor spreading. Mice (4/CTR, 5/treatments) orthotopically implanted with luciferase-expressing human
neuroblastoma cells were treated as reported in the legend of Figure 6 and tumor extension was evaluated by PET after 41 days (A). Glucose consumption maps (white arrows: primary tumor; black arrows: metastases). (B) Number and (C) volume of metastases following treatments. *, p < 0.05: free DXR vs. CTR; * *, p < 0.01: HSYWLRSSL[DXR] vs. free DXR; * * *, p < 0.001: HSYWLRS-SL [DXR] vs. CTR








