This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (CC BY).
ORIGINAL RESEARCH
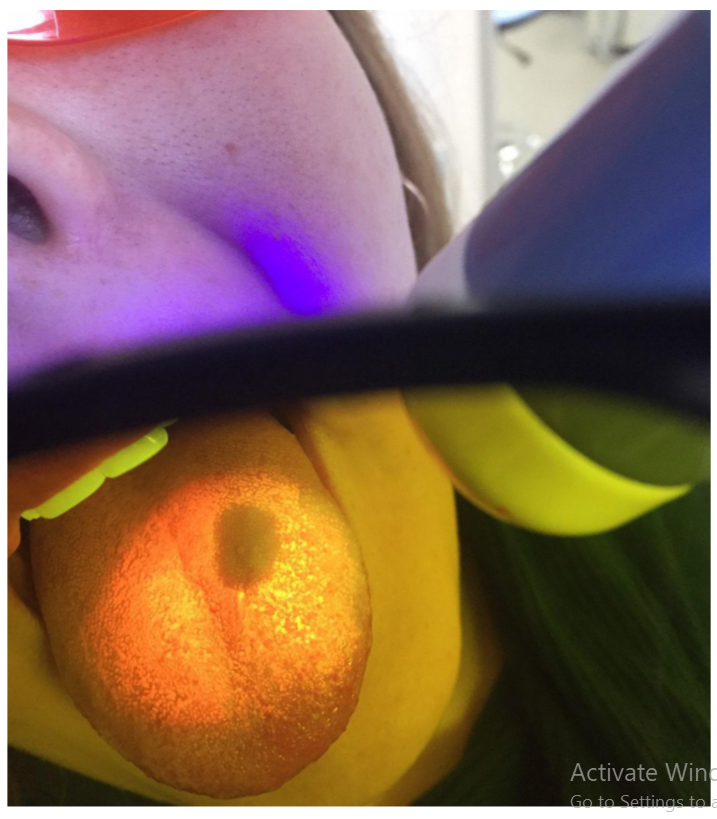
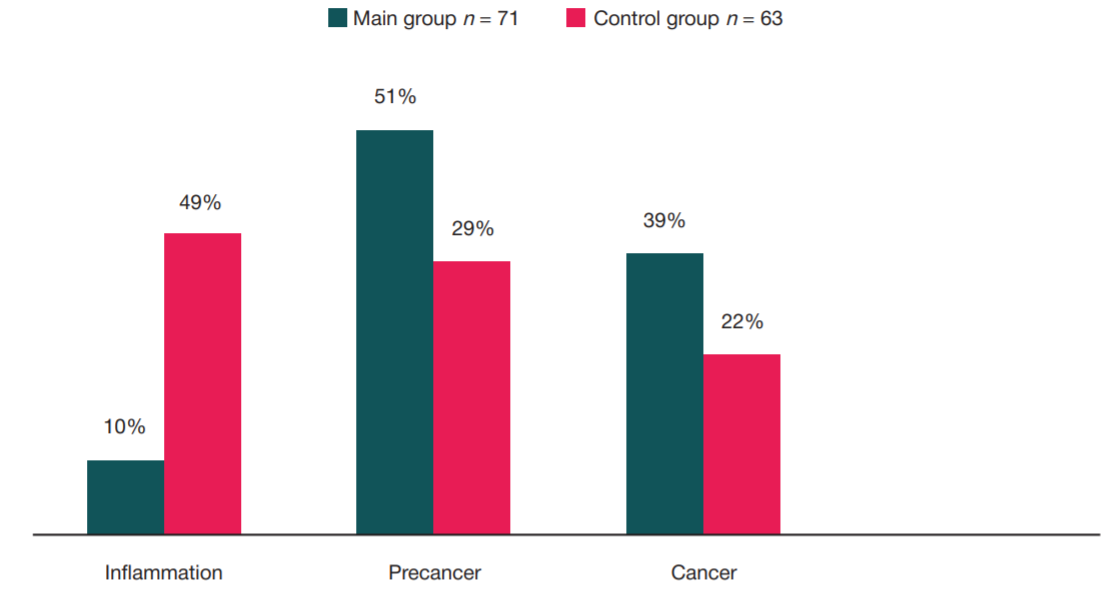
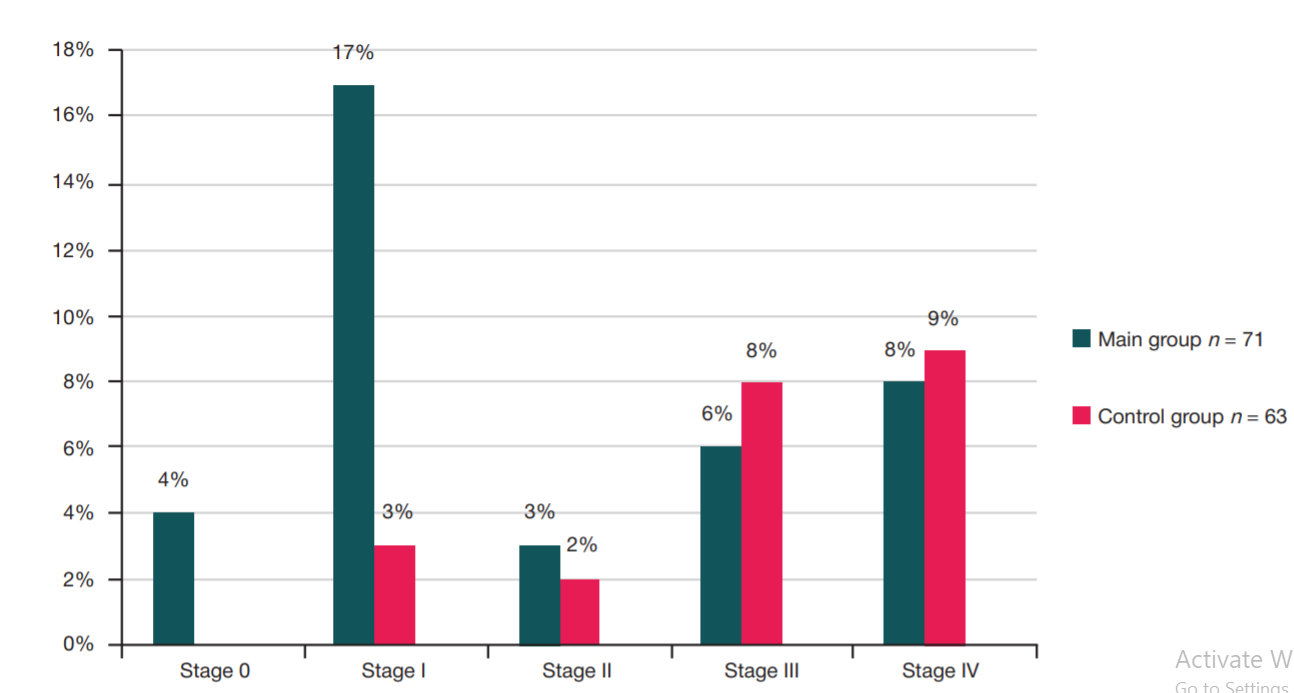
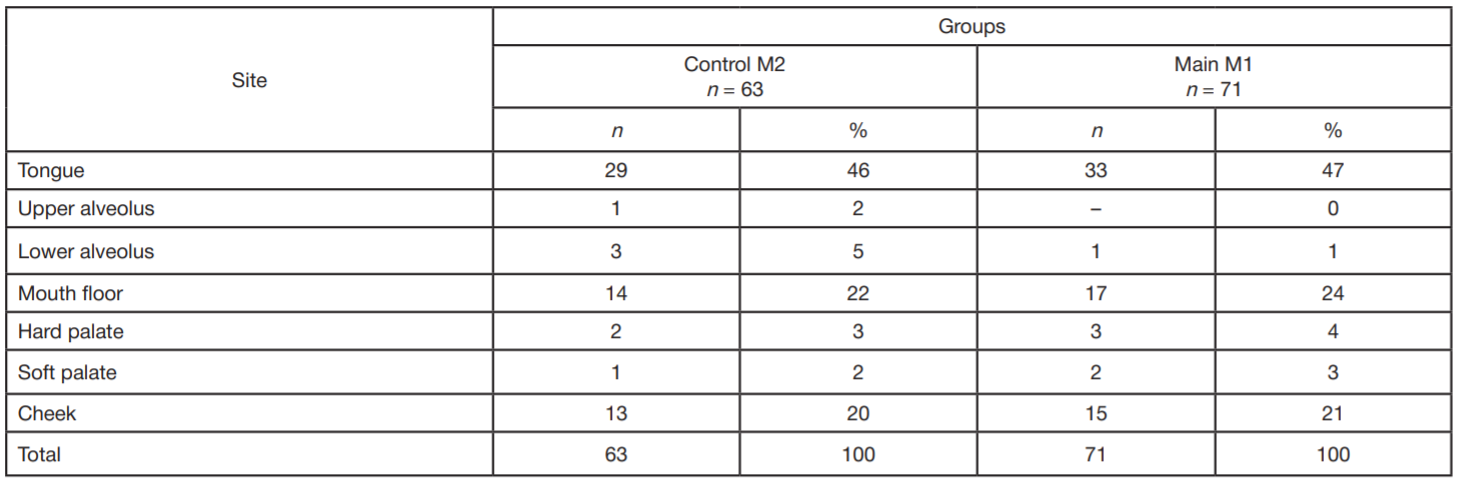
Refinement of noninvasive methods for diagnosing precancer and cancer of oral mucosa in general dental practice
1 Samara State Medical University, Samara, Russia
2 Samara Regional Clinical Cancer Center, Samara, Russia
3 Pirogov Russian National Research Medical University, Moscow, Russia
Correspondence should be addressed: Alexey G. Gabrielyan
Michurina, 138, kv. 85, Samara, 443086; ur.liam@200_leirbag
Author contribution: Postnikov MA — literature analysis; Gabrielyan AG — study planning; Trunin DA, Kopetskiy IS, Eremin DA — analysis of the obtained data; Kaganov OI — manuscript draft; Kirillova VP — analysis of patients’ records; Khamadeeva AM — interpretation of the obtained results; Osokin OV — data acquisition.
Compliance with ethical standards: the study was approved by the Ethics Committee of Samara State Medial University (Protocol № 27 dated February 12, 2018).