This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (CC BY).
ORIGINAL RESEARCH
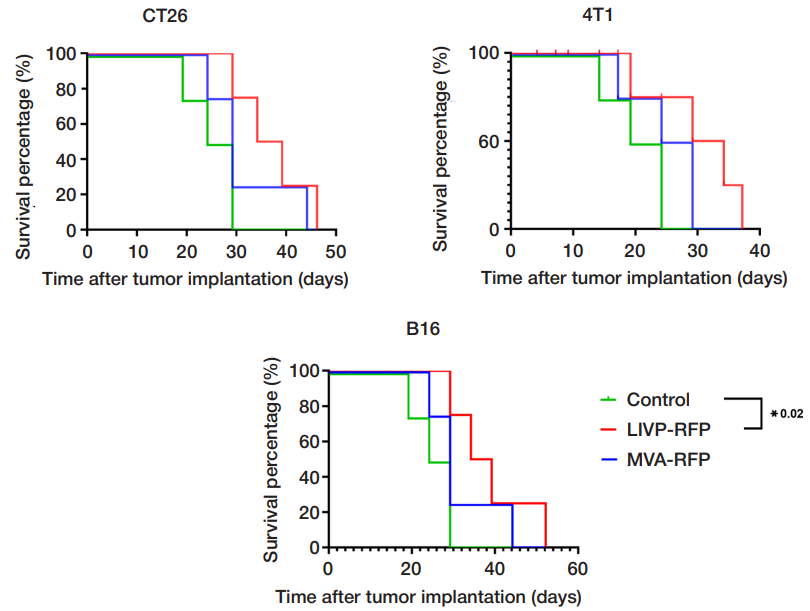
Comparison of the oncolytic activity of recombinant vaccinia virus strains LIVP-RFP and MVA-RFP against solid tumors
1 Engelhardt Institute of Molecular Biology, Russian Academy of Sciences, Moscow, Russia
2 Moscow Institute of Physics and Technology, Dolgoprudny, Russia
3 Engelhardt Institute of Molecular Biology, Russian Academy of Sciences, Moscow, Russia
Correspondence should be addressed: Anastasia V. Lipatova
Vavilova, 32, Moscow, 119991, Russia; moc.liamg@vnaavotapil
Funding: the development of oncolytic viruses and in vitro experiments were supported by the Russian Science Foundation (Russian Science Foundation grant № 20-75-10157); in vivo experiments were also supported by the Russian Science Foundation (Russian Science Foundation grant № 22-64-00057).
Author contribution: Ya Shakiba — literature analysis, pre-analytical work, in vitro and in vivo experiments, analysis and interpretation of data, preparation of figures and graphs; ER Naberezhnaya — in vitro experiments, data analysis and interpretation; D. V. Kochetkov — animal care, data interpretation; GM Yusubaleva — data visualization, manuscript editing; PO Vorobyev — production of preparative quantities of the virus for in vivo studies; VP Baklaushev — study planning, preanalytical stage of work, data analysis; AV Lipatova — research management, design development of recombinant strains, data interpretation, editing the manuscript.
Compliance with ethical standards: the study was approved by the ethics committee of the EIMB RAS (protocol № 3 dated October 27, 2022). Experiments carried out in accordance with Directive 2010/63/EU of the European Parliament and of the Council of Europe on the protection of animals used for research.