This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (CC BY).
ORIGINAL RESEARCH
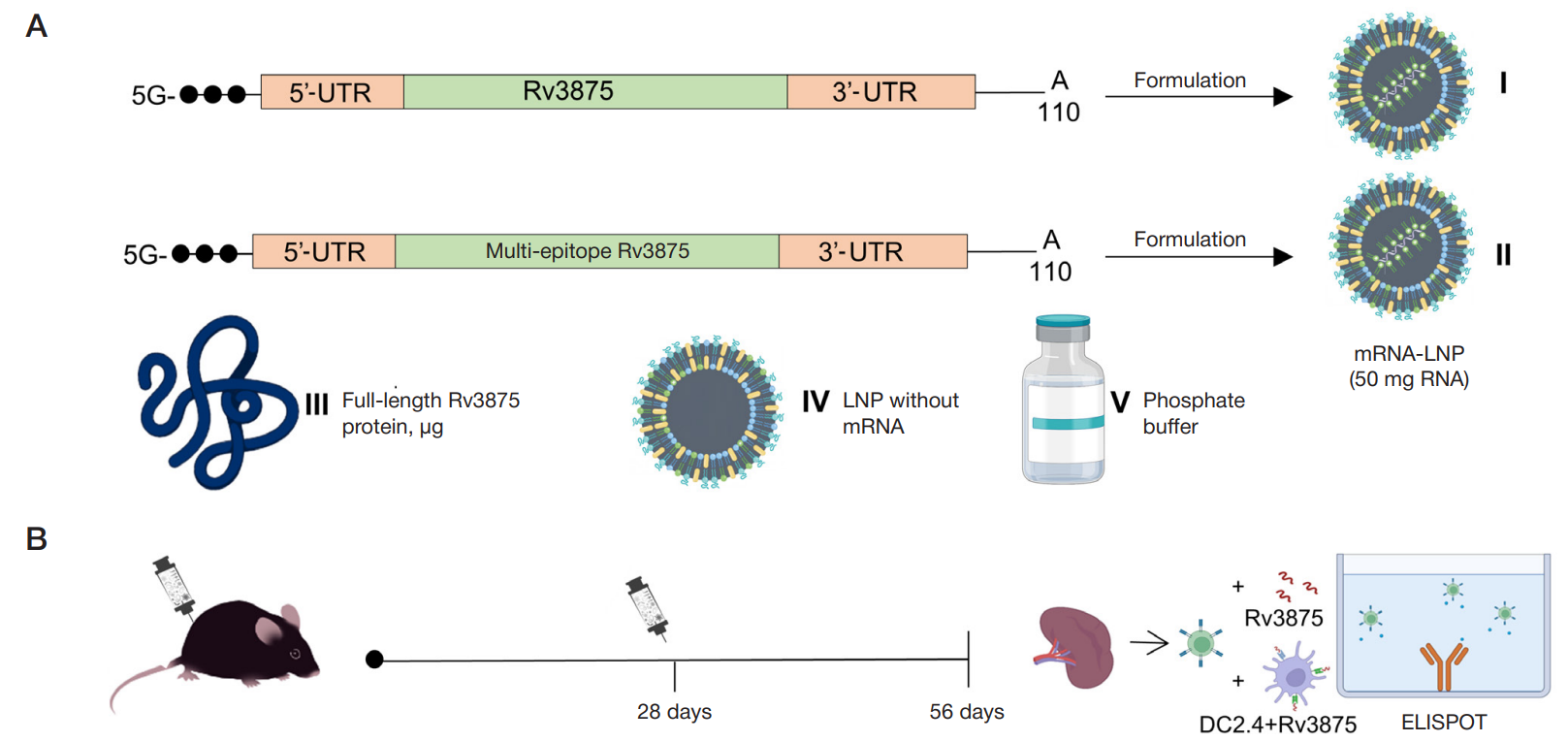
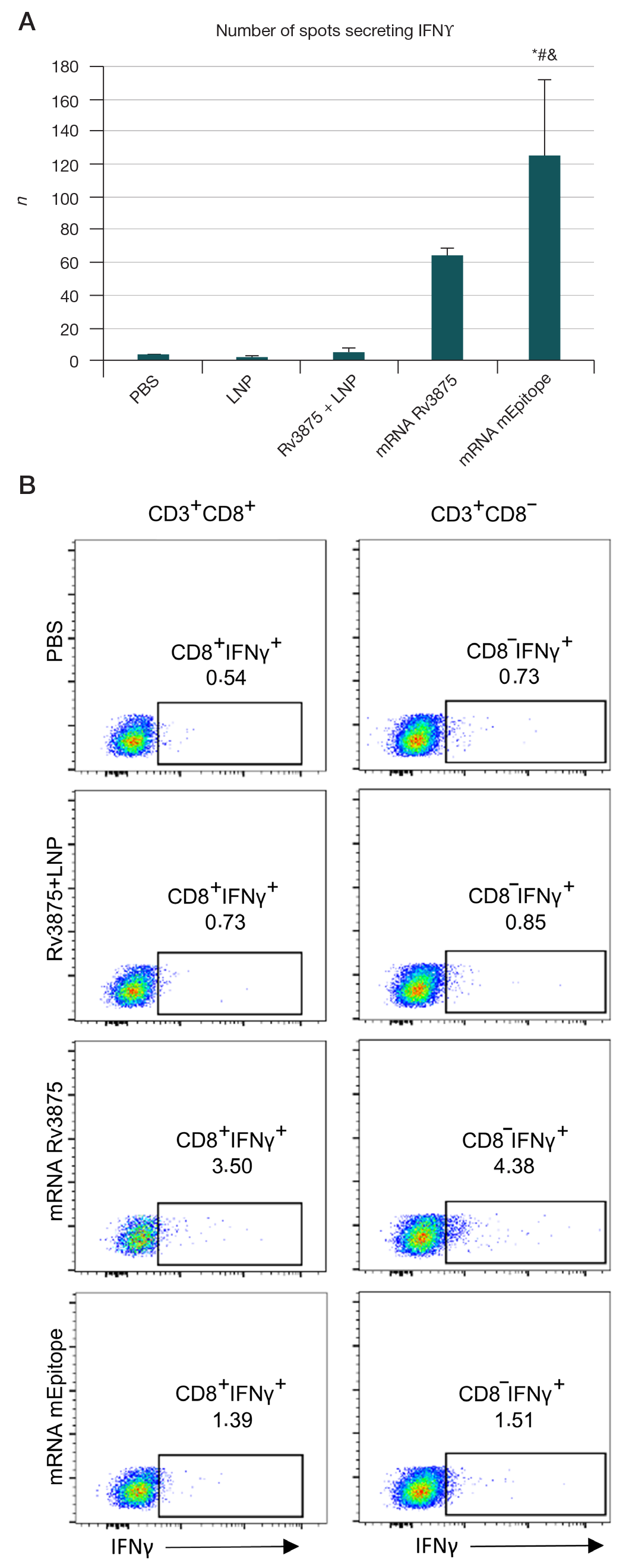
Immunogenicity of full-length and multi-epitope mRNA vaccines for M. Tuberculosis as demonstrated by the intensity of T-cell response: a comparative study in mice
1 Translational Medicine Research Center, Sirius University of Science and Technology (Autonomous Non-Commercial Higher Education Organization), Sirius, Russia
2 Pavlov First Saint Petersburg State Medical University, St. Petersburg, Russia
3 Institute of Cytology and Genetics, Siberian Branch of the Russian Academy of Sciences, Novosibirsk, Russia
Correspondence should be addressed: Vasily V. Reshetnikov
Olympiyskiy prospekt, 1, Sochi, 354340, Russia; ur.hepsuitnalat@vv.vokintehser
Funding: the study was supported by the Ministry of Science and Higher Education of the Russian Federation (agreement № 075-10-2021-113, unique project identifier RF----193021X0001).
Acknowledgements: the authors express their gratitude to the staff of the Sirius University: Terenin IM for in vitro transcription, Zaborova OV for the formulation of mRNA into lipid nanoparticles, Gavrilyak MA and Golovin EV for purification and characterization of the Rv3875 protein; Shevyrev DV for setting up the intracellular staining experiment and Sitikova VA for assistance in the context of the animal experiment.
Author contribution: Vasileva OO, Tereschenko VP — work with the cells, data analysis, article authoring, figures authoring; Krapivin BN, Pateev II, Rybtsov SA — work with the cells, animal experiment, data analysis; Muslimov AR, Kukushkin IS — design of structures, cloning; Ivanov RA, Reshetnikov VV — text editing, data analysis, project coordination.
Compliance with the ethical standards: animal study was approved by the Ethics Committee of the Institute of Cytology and Genetics (2022); carried out in accordance with the laboratory animals care guidelines (2010), Directive 2010/63/EU of the European parliament and of the Council on the protection of animals used for scientific purposes (2010), Good Laboratory Practice guidelines (2016).