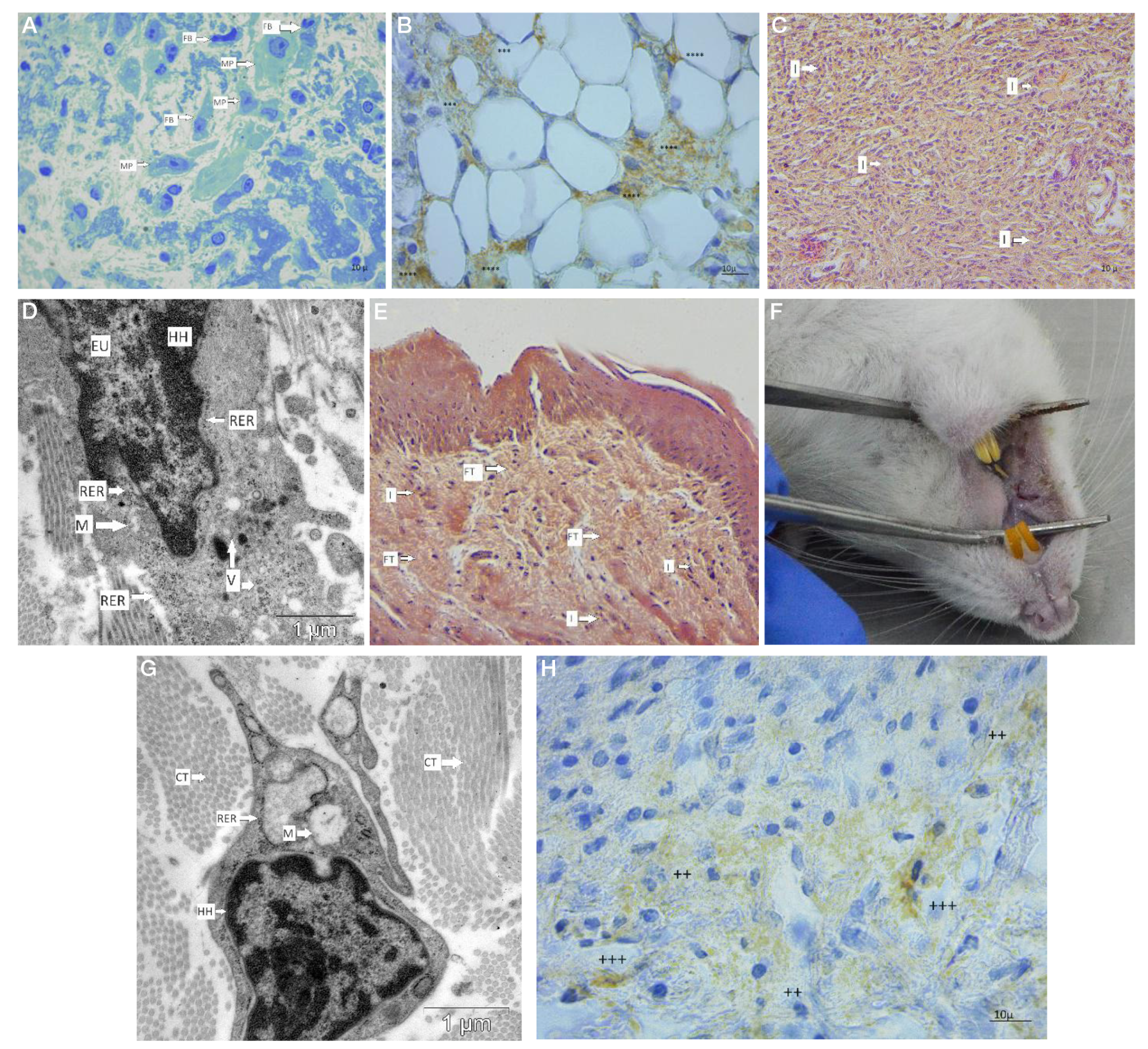
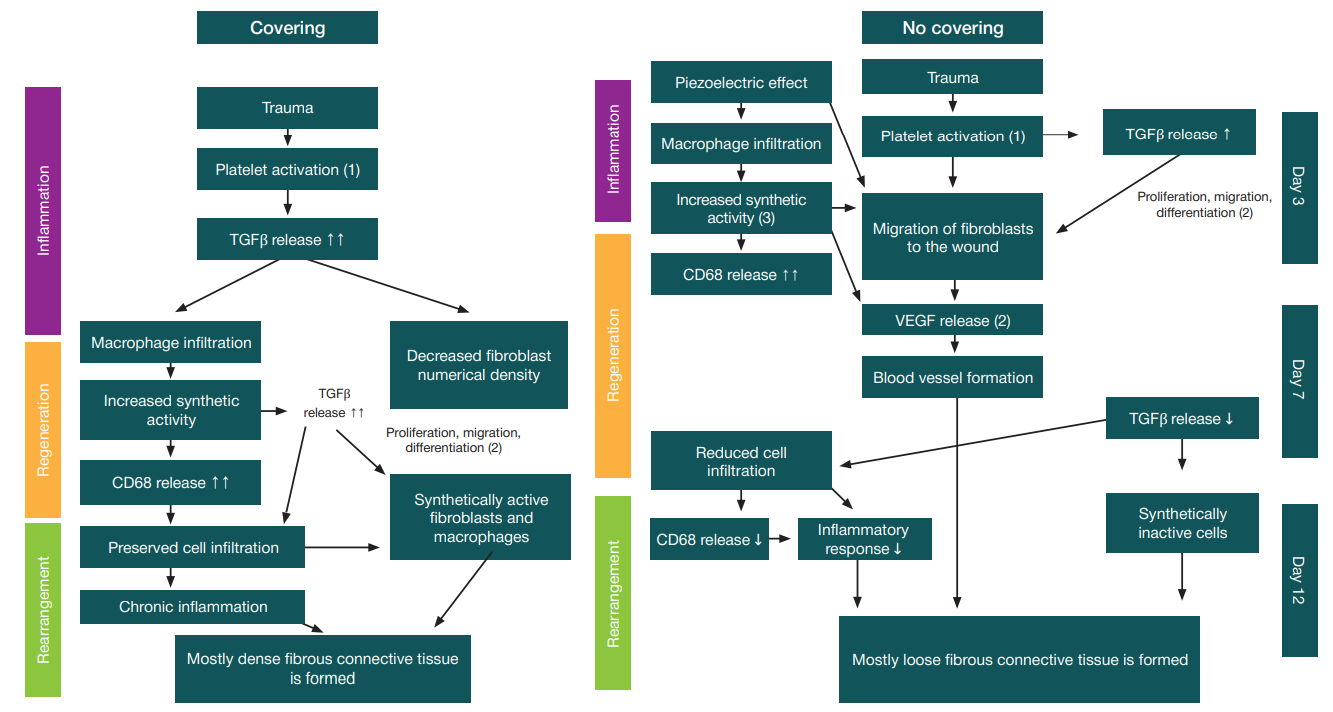
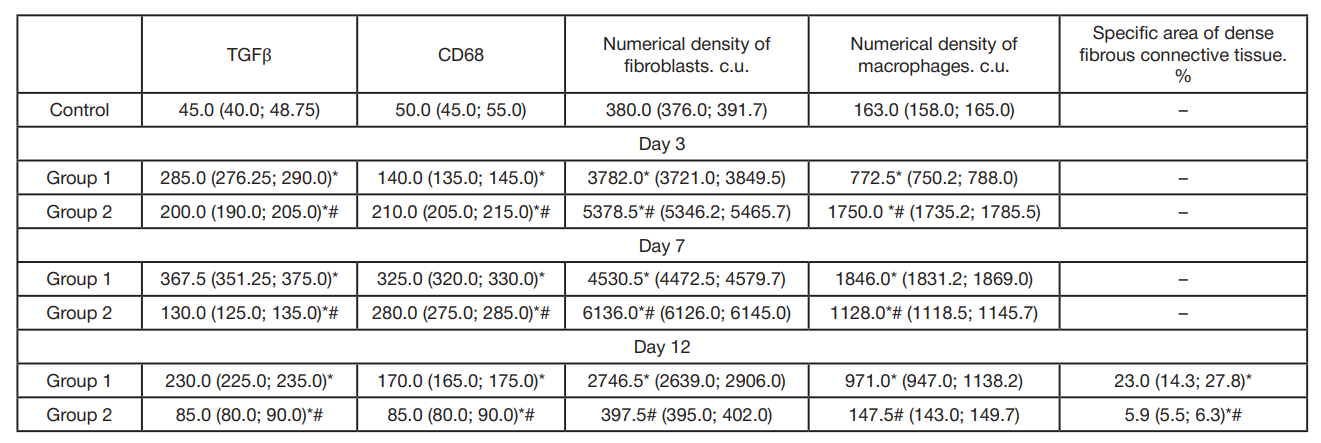
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (CC BY).
ORIGINAL RESEARCH
Effects of biocompatible piezoelectric membranes on the development of fibrosis associated with the oral mucosal wound regeneration
1 Siberian State Medical University, Tomsk, Russia
2 Tomsk Polytechnic University, Tomsk, Russia
3 Montana State University, Bozeman, MT, USA
Correspondence should be addressed: Anastasiia D. Koniaeva
Moskovskij trakt, 2, Tomsk, 634034, Russia; moc.liamg@59aynokaysa
Funding: the study was supported by the RSF (research project No. 23-25-00346)
Author contribution: Koniaeva AD, Varakuta EYu, Bolbasov EN, Stankevich KS — study concept and design; Koniaeva AD, Leiman AE, Kormashov GM, Fedosova MV — data acquisition and processing; Koniaeva AD, Varakuta EYu — manuscript writing; Koniaeva AD, Varakuta EYu, Bolbasov EN, Stankevich KS — manuscript editing.
Compliance with ethical standards: the study was approved by the IACUC of the Siberian State Medical University (protocol No. 11-1 dated 12 July 2022). Rats were handled in accordance with the Directive 2010/63/EU of the European Parliament and of the Council on the protection of animals used for scientific purposes dated 22 September 2010.