This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (CC BY).
ORIGINAL RESEARCH
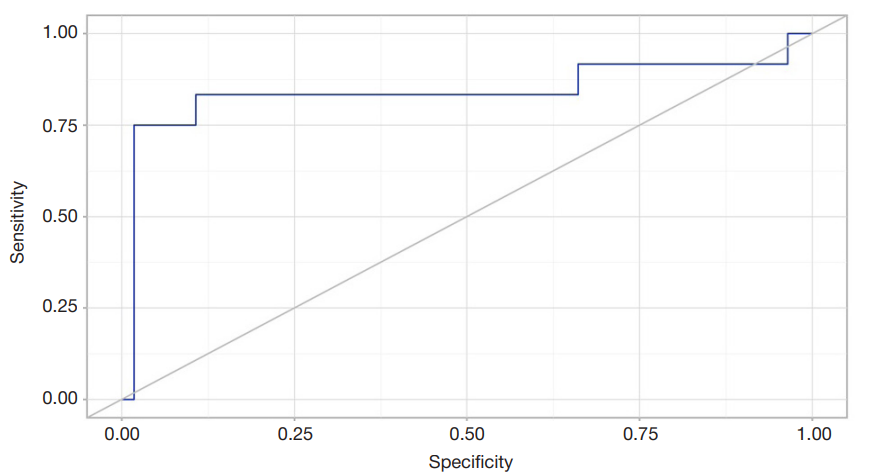
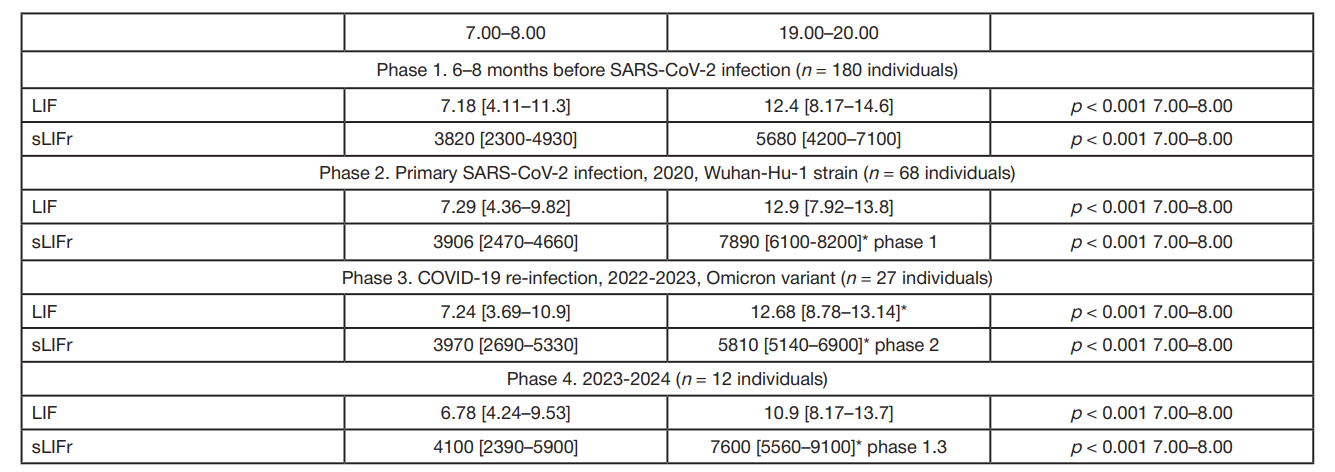
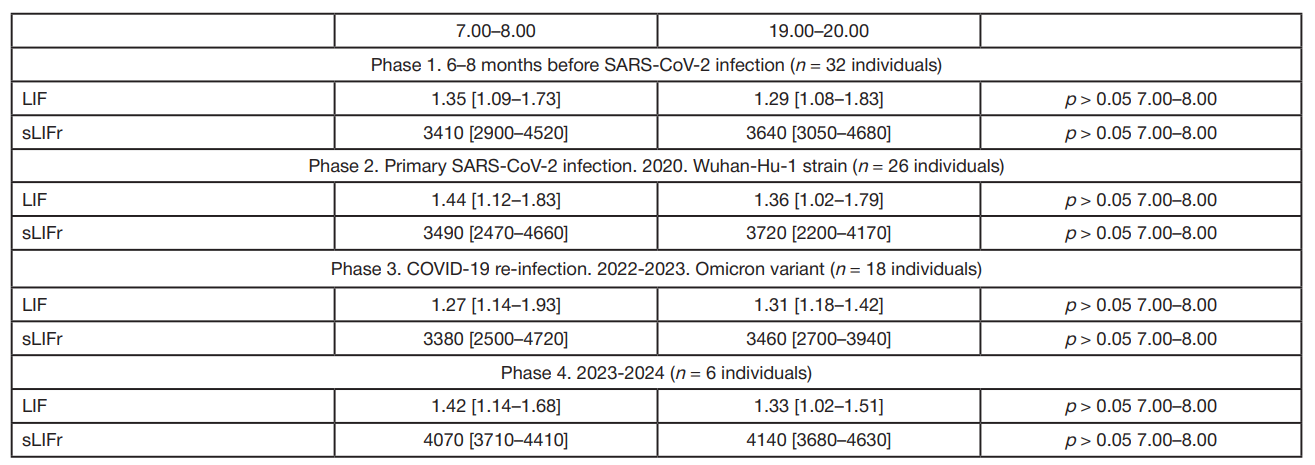
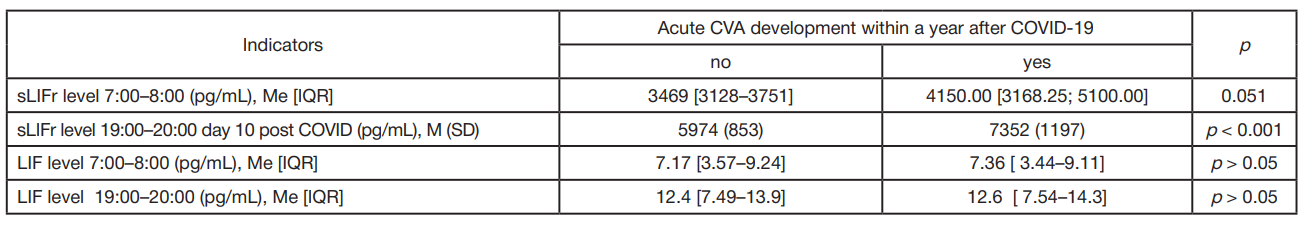
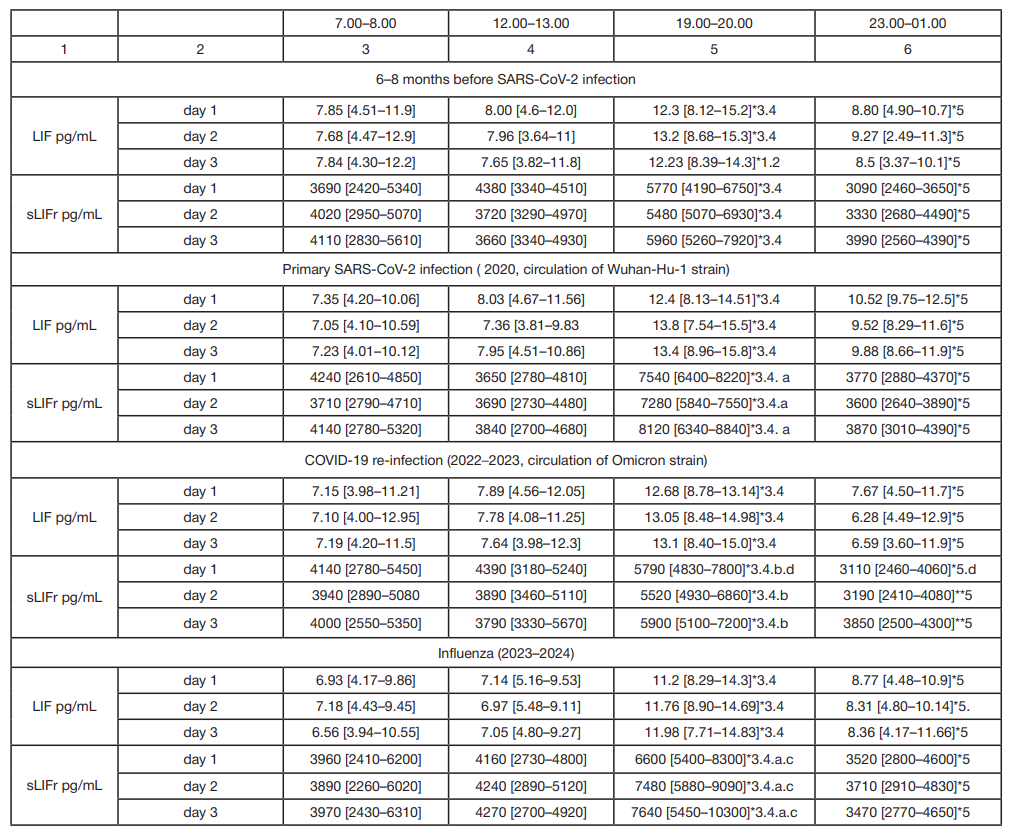
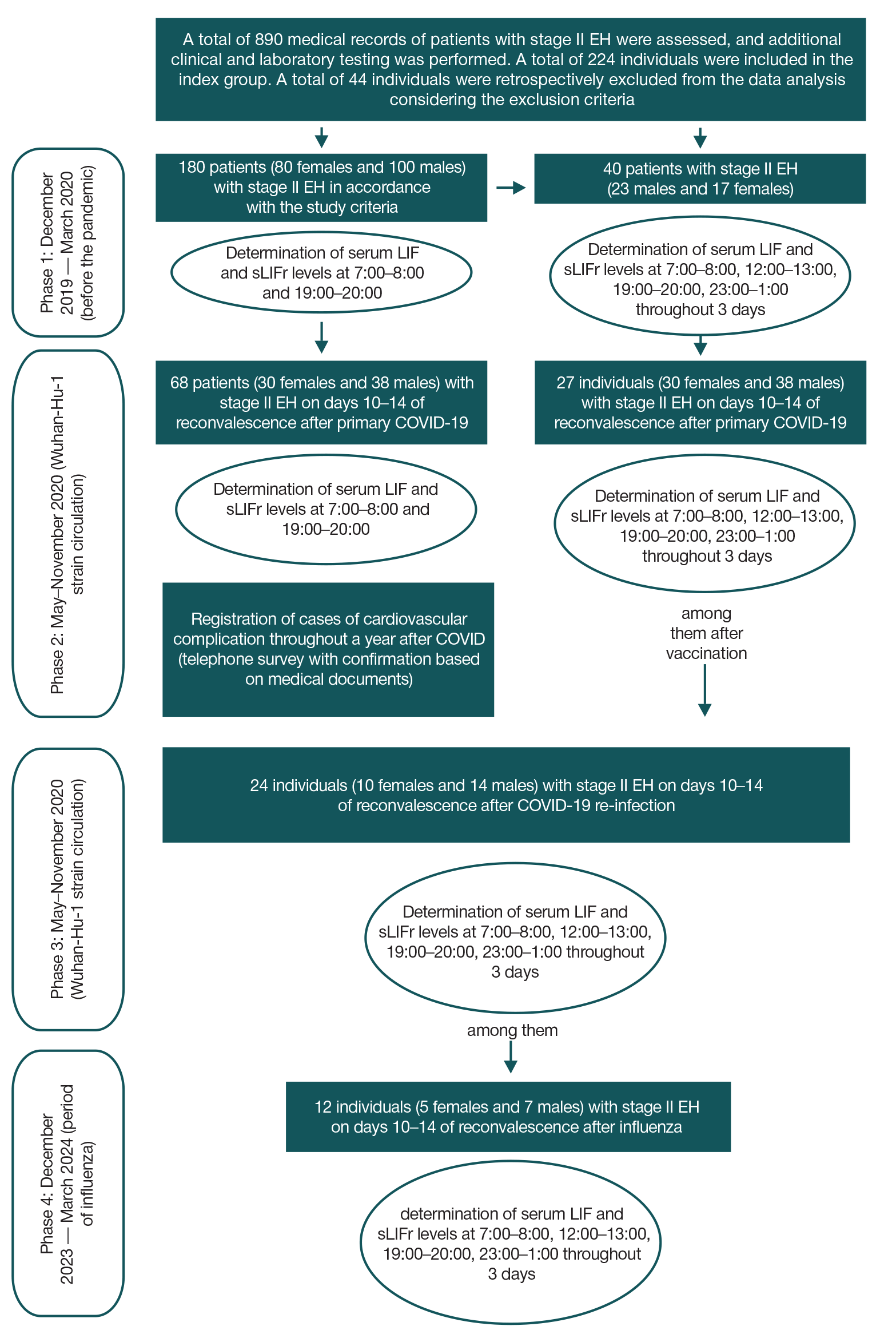
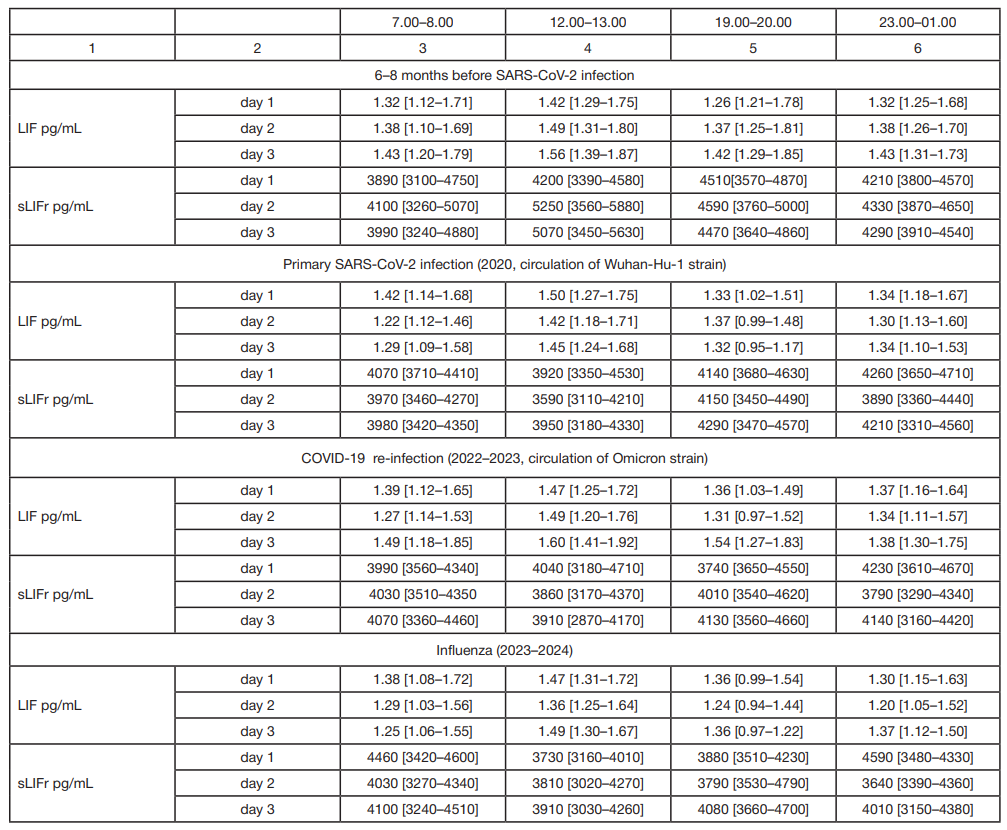
LIF and sLIFr alterations during reconvalescence (novel coronavirus infection, influenza) in patients with essential hypertension
1 National Research Mordovia State University, Saransk, Russia
2 State Research and Development Institute of High Purity Biologicals of the Federal Medical and Biological Agency, Saint-Petersburg, Russia
Correspondence should be addressed: Olga A. Radaeva
Ulyanov, 26а, Saransk, 430032, Russia; ur.liam@demaveadar
Funding: the study was supported by the RSF grant “Analysis of changes in circadian rhythms of cytokine synthesis in the blood of patients with essential arterial hypertension as a predictor of the development of cardiovascular complications”, No. 23-25-00147.
Author contribution: Radaeva OA — developing the study design, analysis of the results, manuscript formatting; Simbirtsev AS — phrasing of the aim of the study, manuscript editing; Kostina YuA — laboratory testing, manuscript formatting; Iskandyarova MS — literature review, manuscript editing; Negodnova EV — literature review, patient follow-up; Solodovnikova GA — statistical data processing; Eremeev VV — manuscript editing; Krasnoglazova KA, Babushkin IO — statistical processing of the 6-month follow-up data.
Compliance with ethical standards: the study was approved by the Ethics Committee of the Ogarev Mordovia State University (protocol No. 12 dated 14 December 2008, additional protocol No. 85 dated 27 May 2020). The informed consent was submitted by all patients. Biomaterial (blood) was collected for further testing considering provisions of the WMA Declaration of Helsinki (2013) and the protocol of the Convention on Human Rights and Biomedicine developed by the Council of Europe (1999) considering supplementary protocol of the Convention on Human Rights and Biomedicine in the field of biomedical research (2005).