This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (CC BY).
ORIGINAL RESEARCH
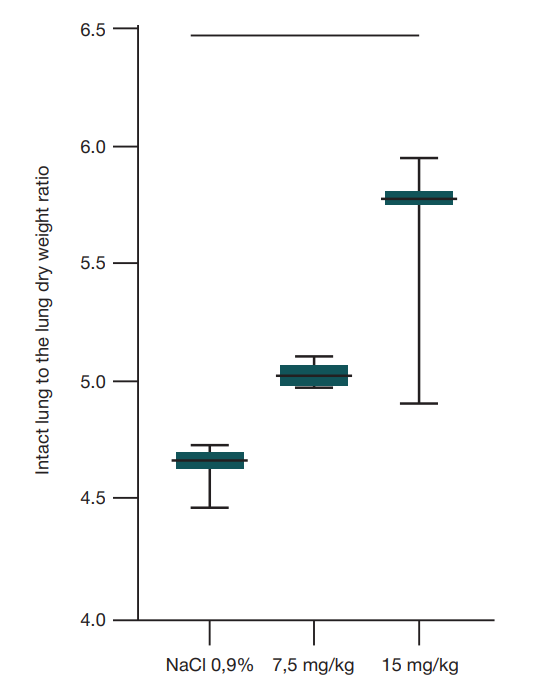
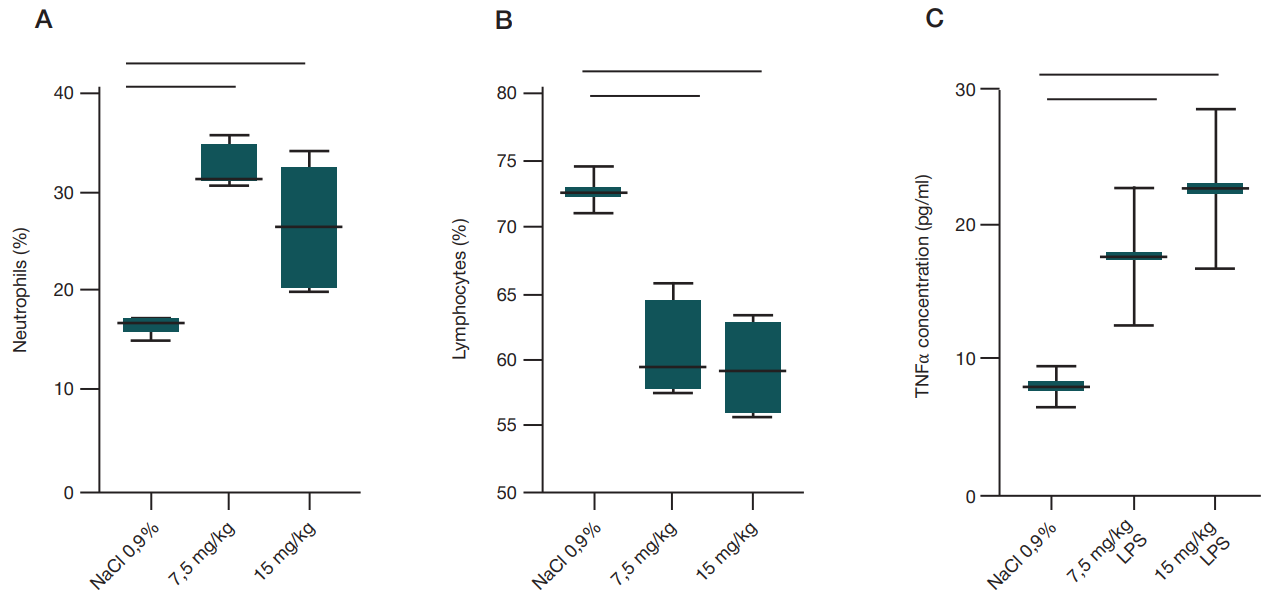
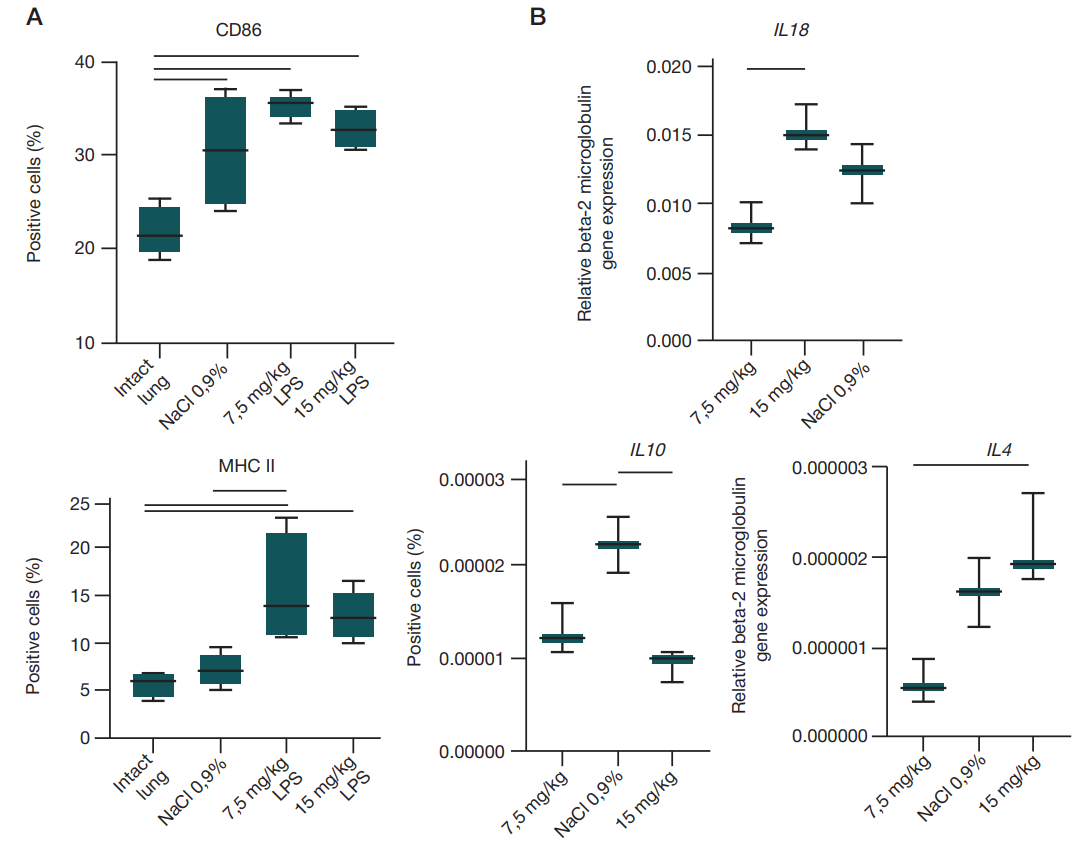
Intranasal lipopolysaccharide administration to Sprague-Dawley rats as a biomodel of acute respiratory distress syndrome
1 Patrice Lumumba Peoples' Friendship University of Russia, Moscow, Russia
2 Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Moscow, Russia
3 Petrovsky National Research Centre of Surgery, Moscow, Russia
Correspondence should be addressed: Victoria V. Kiseleva
Oparina, 4, Moscow, 117997, Russia; moc.liamg@1991.avosonruk.airotciv
Funding: the study was supported by the Russian Science Foundation (grant No. 24-25-00203).
Acknowledgements: the authors would like to thank D.A. Areshidze, C. Sci. Biol., Head of the Cellular Pathology Laboratory of the Avtsyn Institute of Human Morphology for performing the complete blood count test in animals.
Author contribution: Kiseleva VV — experimental design and procedure, analysis of the results, manuscript writing; Vishnyakova PA — advice on the experimental procedure, material resources, editing; Kosyreva AM — advice on the experimental procedure, editing; Kananykhina EYu, Emelianov II — animal handling; Elchaninov AV — advice on the experimental procedure, material resources, editing; Fatkhudinov TH — material resources for the study.
Compliance with ethical standards: the study was approved by the Ethics Committee of the Avtsyn Institute of Human Morphology (protocol No. 21 dated 29 March 2019). Animals were handled in accordance to the ARRIVE guidelines and the Directive ЕС 2010/63/EU on the protection of animals used for scientific purposes.