This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (CC BY).
ORIGINAL RESEARCH
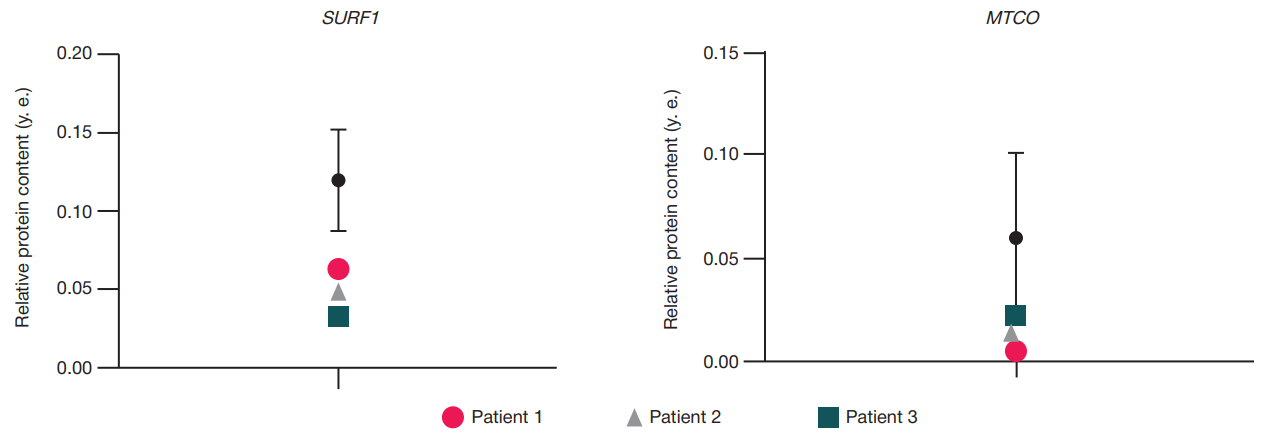
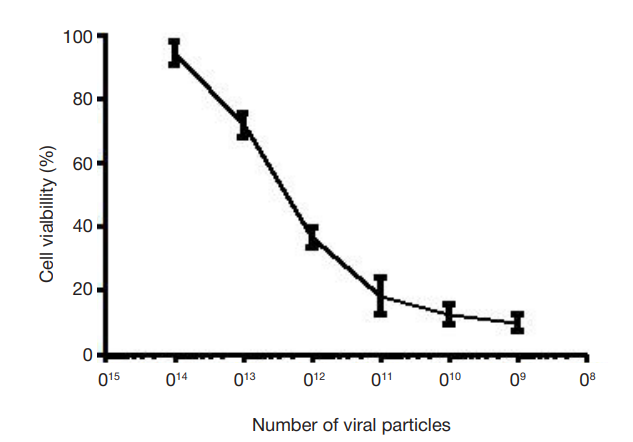
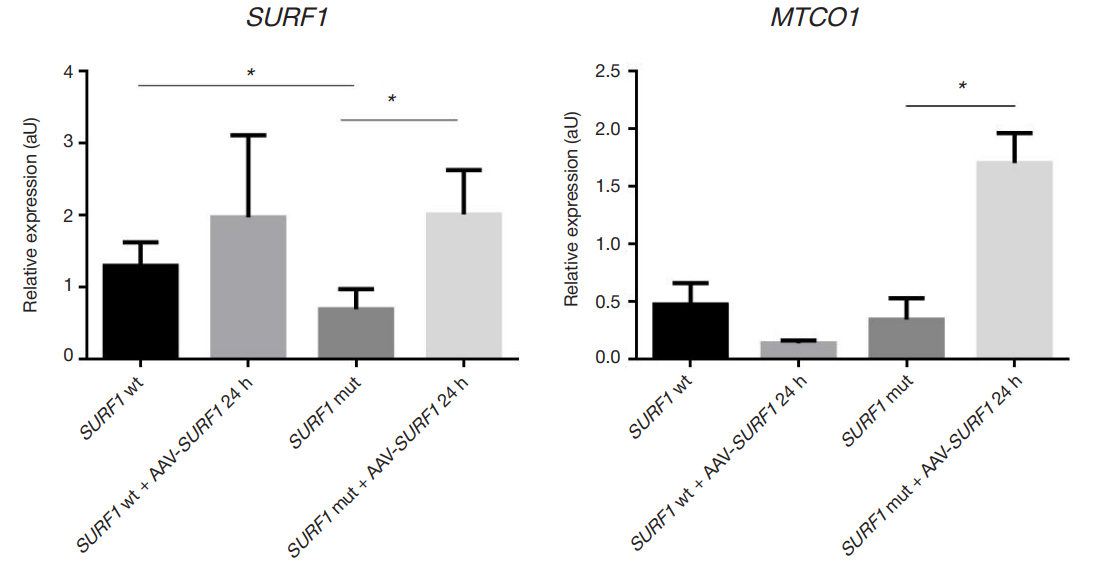
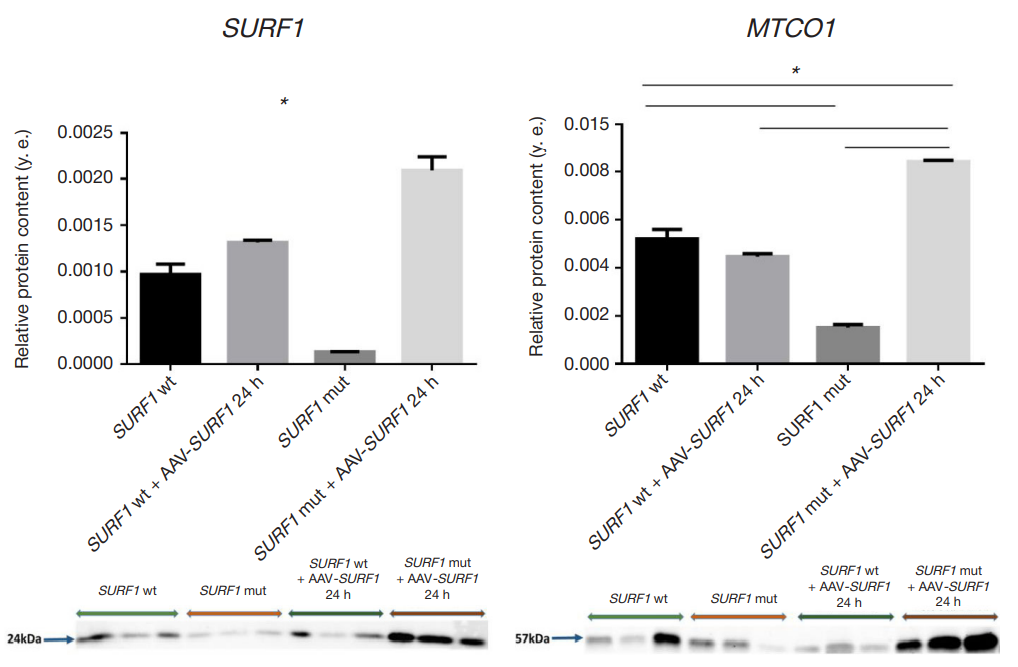
Expression of MTCO1 in cell cultures from patients with Leigh syndrome under the action of AAV9-SURF1
1 Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Moscow, Russia
2 Belozersky Research Institute of Physico-Chemical Biology at Lomonosov Moscow State University, Moscow, Russia
3 Filatov Clinical Institute of Child Health, Sechenov First Moscow State Medical University, Moscow, Russia
4 Pirogov Russian National Research Medical University, Moscow, Russia
Correspondence should be addressed: Mikhail A. Adrianov
Academica Oparina, 4, 117997, Moscow, Russia, moc.liamg@hcstil.ay
Funding: The study was conducted under state assignment number EGISU 125022002694-7, "Development of gene therapy drugs based on viral delivery for the treatment of hemophilia, Leigh syndrome, and glycogen storage disease type Ia." The project was conducted at the FSBI «National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I.Kulakov», Ministry of Health of the Russian Federation.
Acknowledgements: The authors thank A. M. Gamisoniya and N. M. Marycheva for the help in the research.
Author contribution: Adrianov MA — gene expression assessment, manuscript preparation; Gautier MS, Degtyareva AV, Ushakova LV — clinical work with patients; Rastorguev SM, Simonov VS — construction and preparation the AAV vector; Marey MV — protein expression assessment, fibroblasts cultivation, transfection, and the viral construct toxicity assessment, microscopy; Manukhova LA — sample processing, protein expression assessment, data processing and statistical analysis; Vysokikh MY — overall supervision, the research coordination, writing the manuscript.
Compliance with ethical standards: all procedures with patients were carried out in accordance with the rules of Ethics Committee of the Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology (Protocol No. 10, dated November 28, 2024). Voluntary informed consent was obtained from all study participants regarding their inclusion in the study and the use of their biological materials.