This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (CC BY).
ORIGINAL RESEARCH
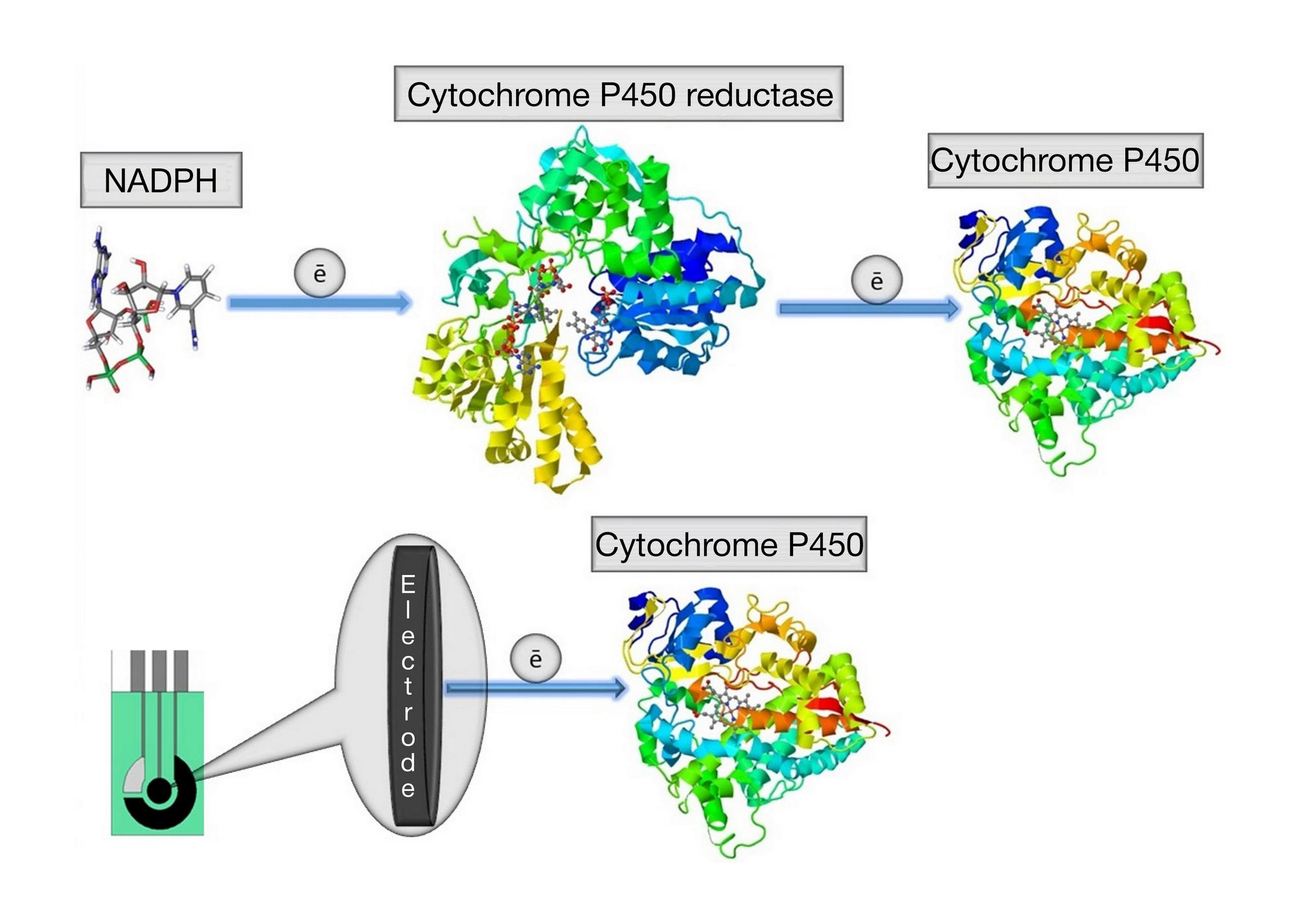
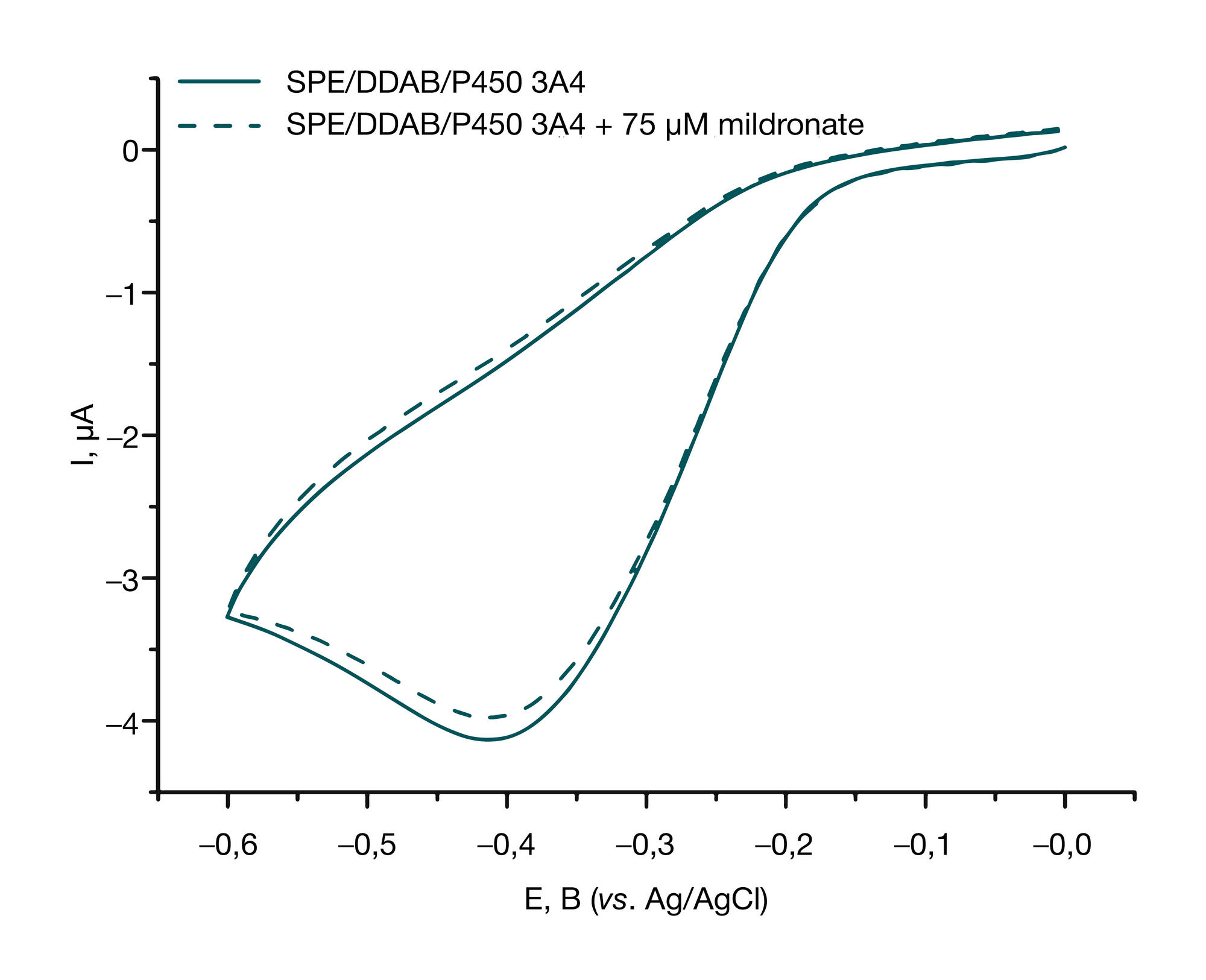
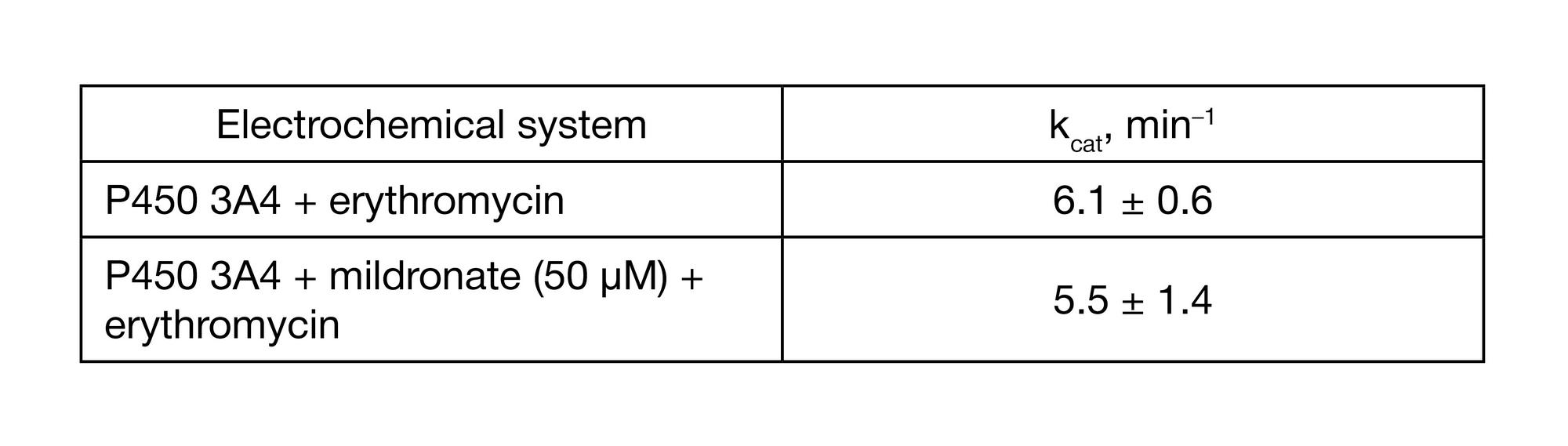
Analysis of mildronate effect on the catalytic activity of cytochrome Р450 3А4
1 Laboratory of Bioelectrochemistry, Department of Personalized Medicine,
Institute of Biomedical Chemistry, Moscow, Russia
2 Department of Biochemistry, Biomedical Faculty,
Pirogov Russian National Research Medical University, Moscow, Russia
3 Department of Clinical Pharmacology and Propaedeutics of Internal Diseases, Faculty of General Medicine,
I. M. Sechenov First Moscow State Medical University, Moscow, Russia
4 Institute of Bioorganic Chemistry of the National Academy of Sciences, Minsk, Republic of Belarus
Correspondence should be addressed: Victoria Shumyantseva
ul. Pogodinskaya, d. 10, Moscow, Russia, 119121; ur.liam@avestnaymuhs_v
Funding: this work was conducted under the Federal Fundamental Scientific Research Program for 2013–2020.