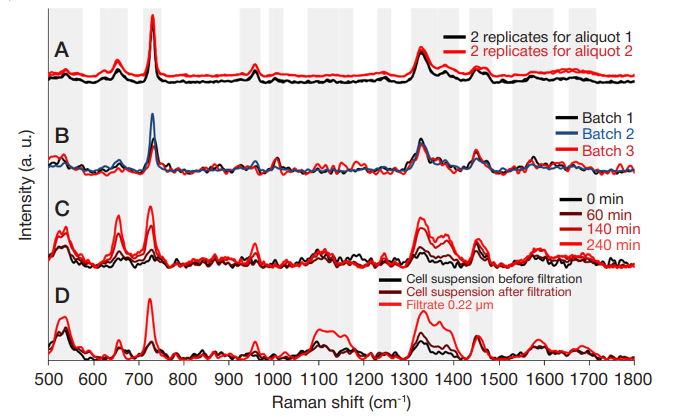
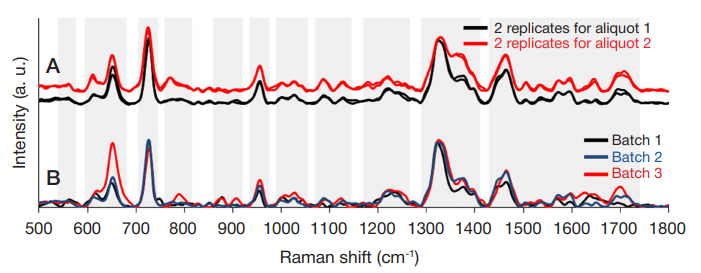
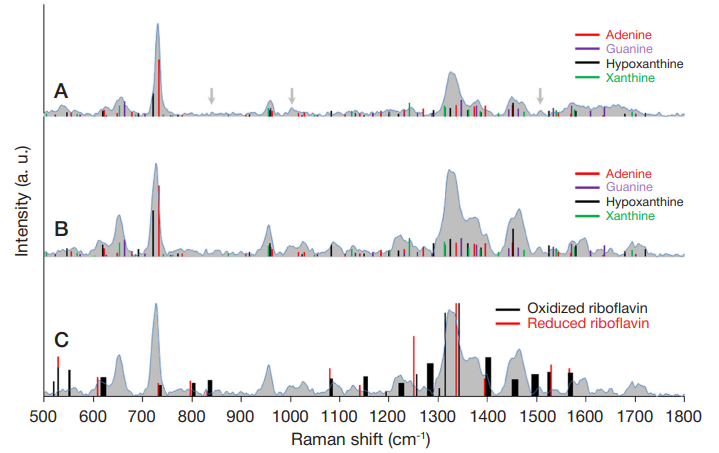
ISSN Print 2500–1094
ISSN Online 2542–1204
BIOMEDICAL JOURNAL OF PIROGOV UNIVERSITY (MOSCOW, RUSSIA)

1 Faculty of Chemistry, Lomonosov Moscow State University
2 Emanuel Institute of Biochemical Physics of the Russian Academy of Sciences, Moscow, Russia
Correspondence should be addressed: Evgeniy G. Evtushenko
Leninskie gory, 1 bl. 3, Moscow, 119991; ur.usm.mehc.emyzne@oknehsutve