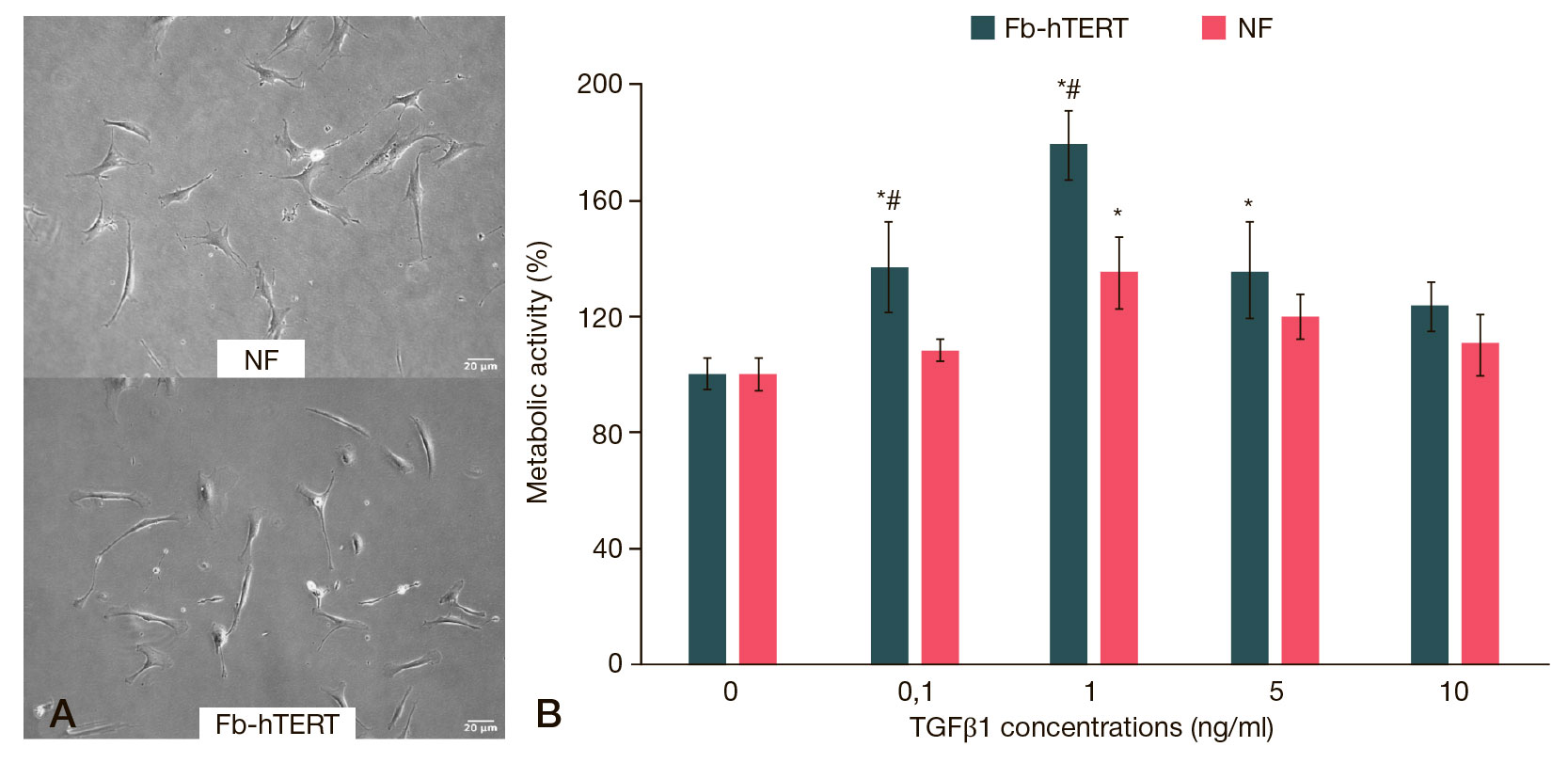
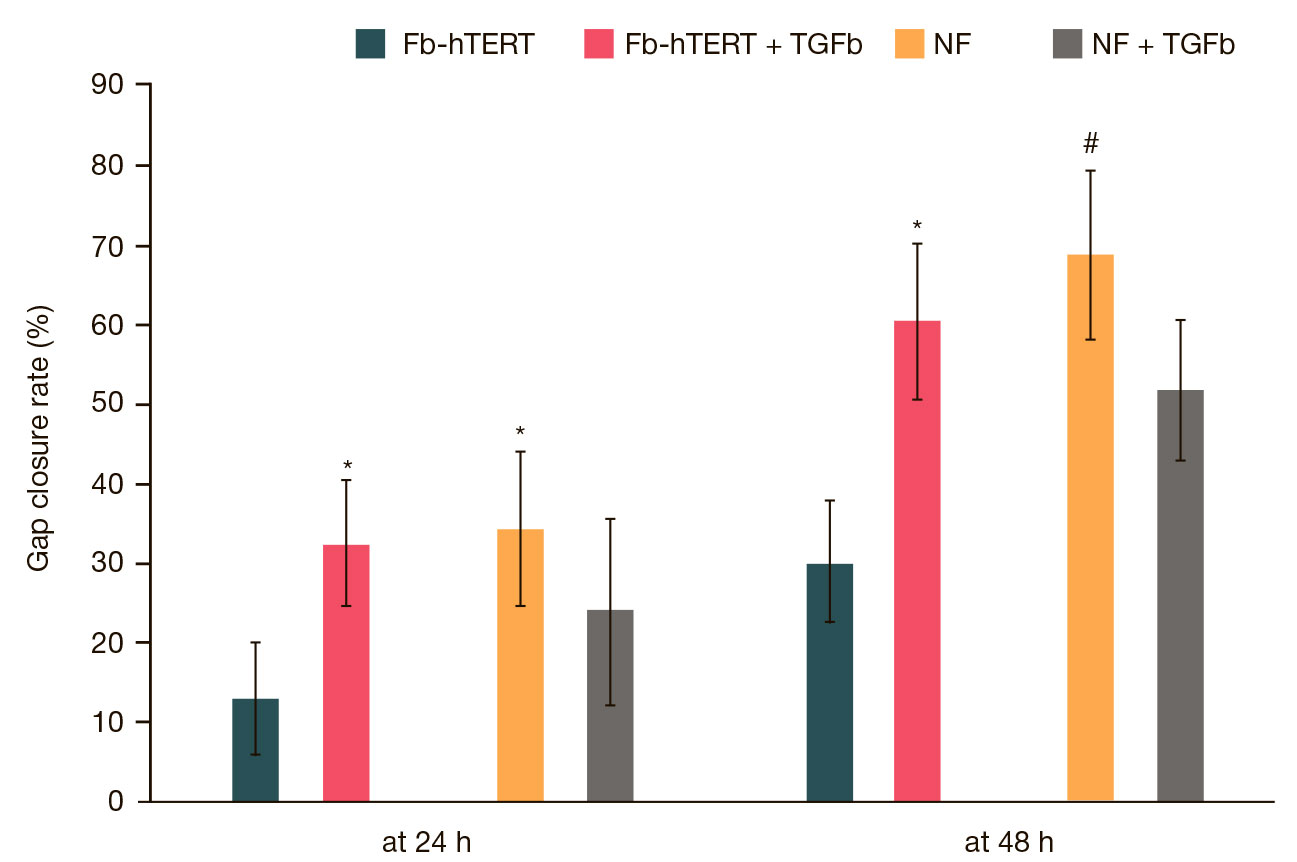
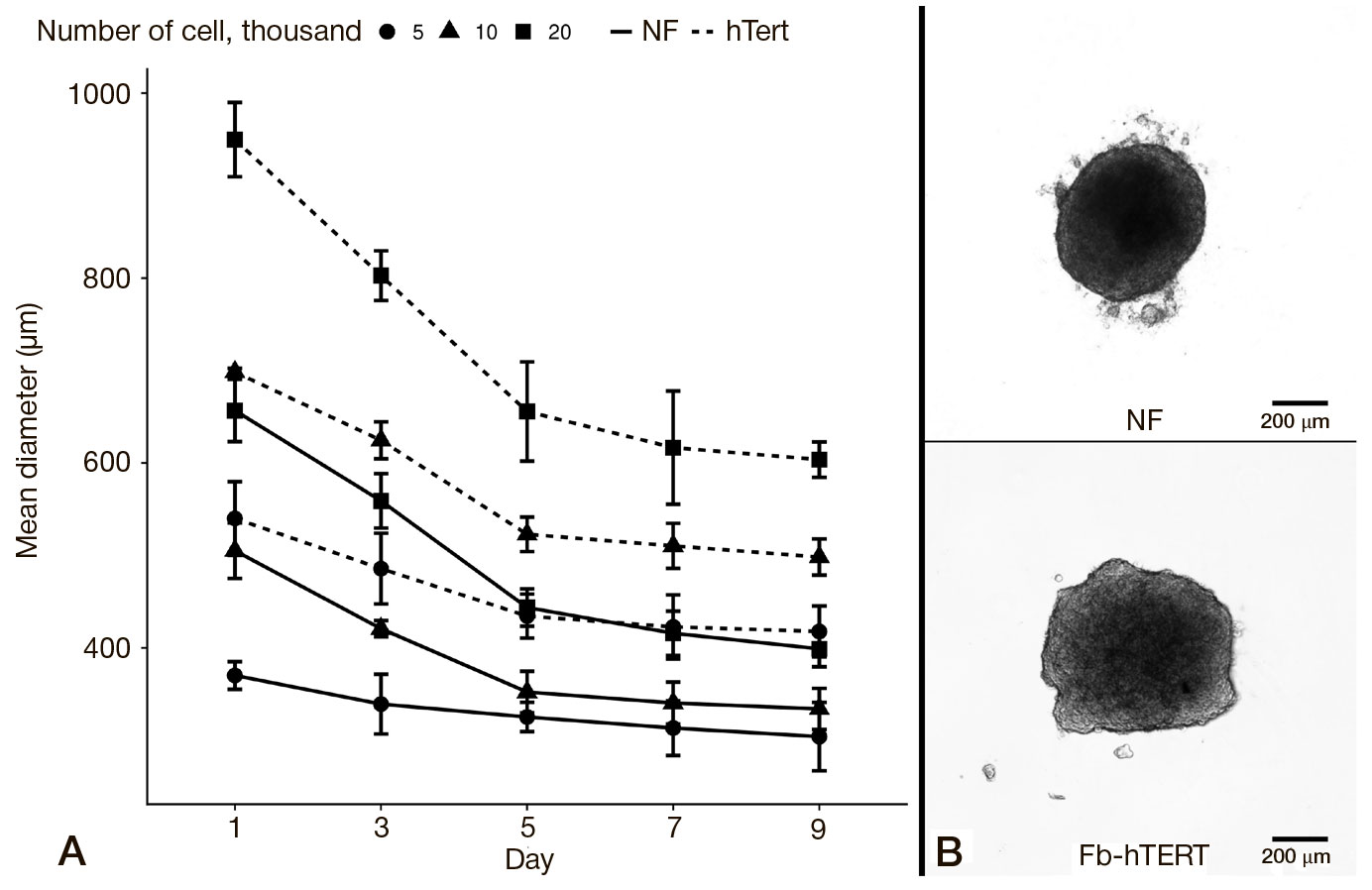
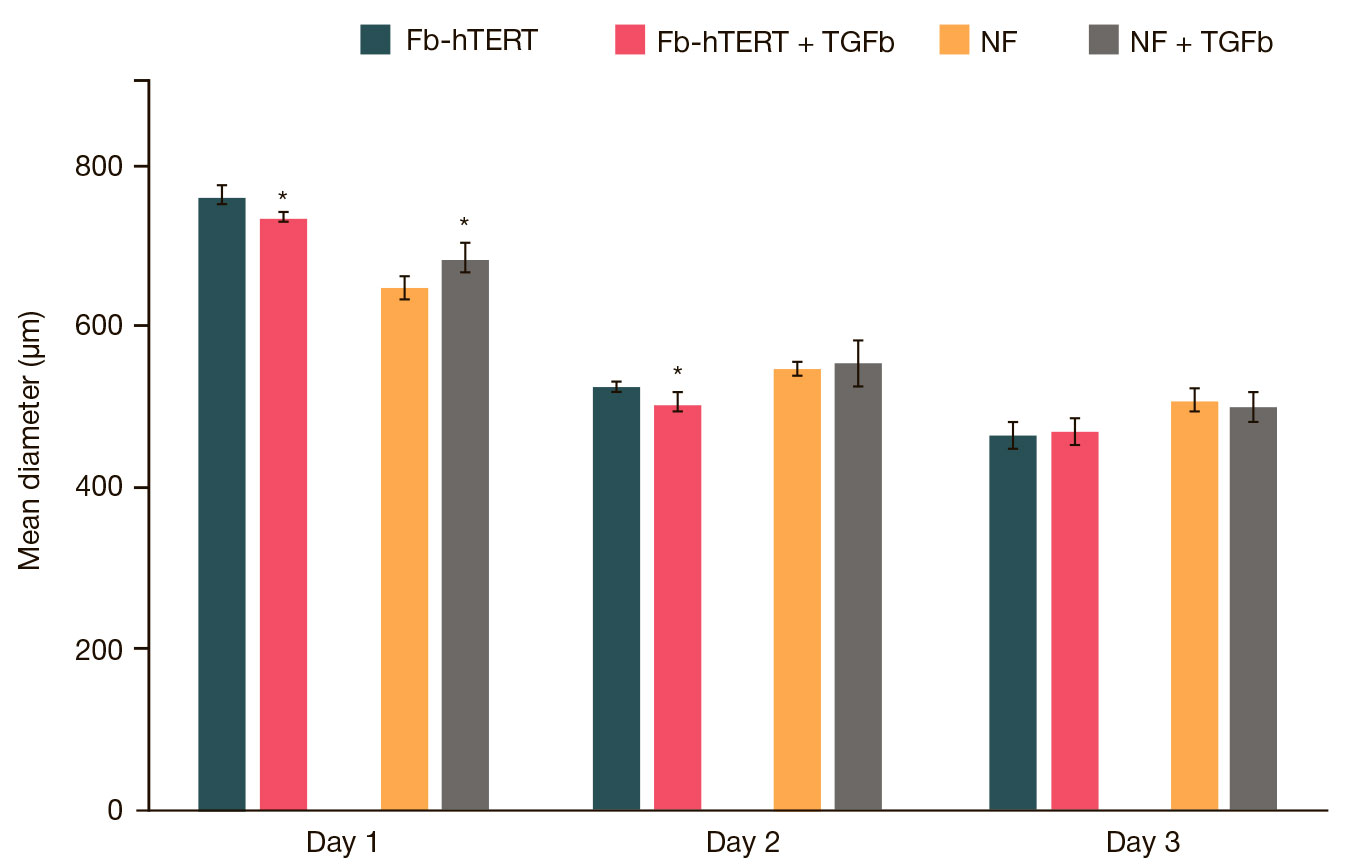
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (CC BY).
ORIGINAL RESEARCH
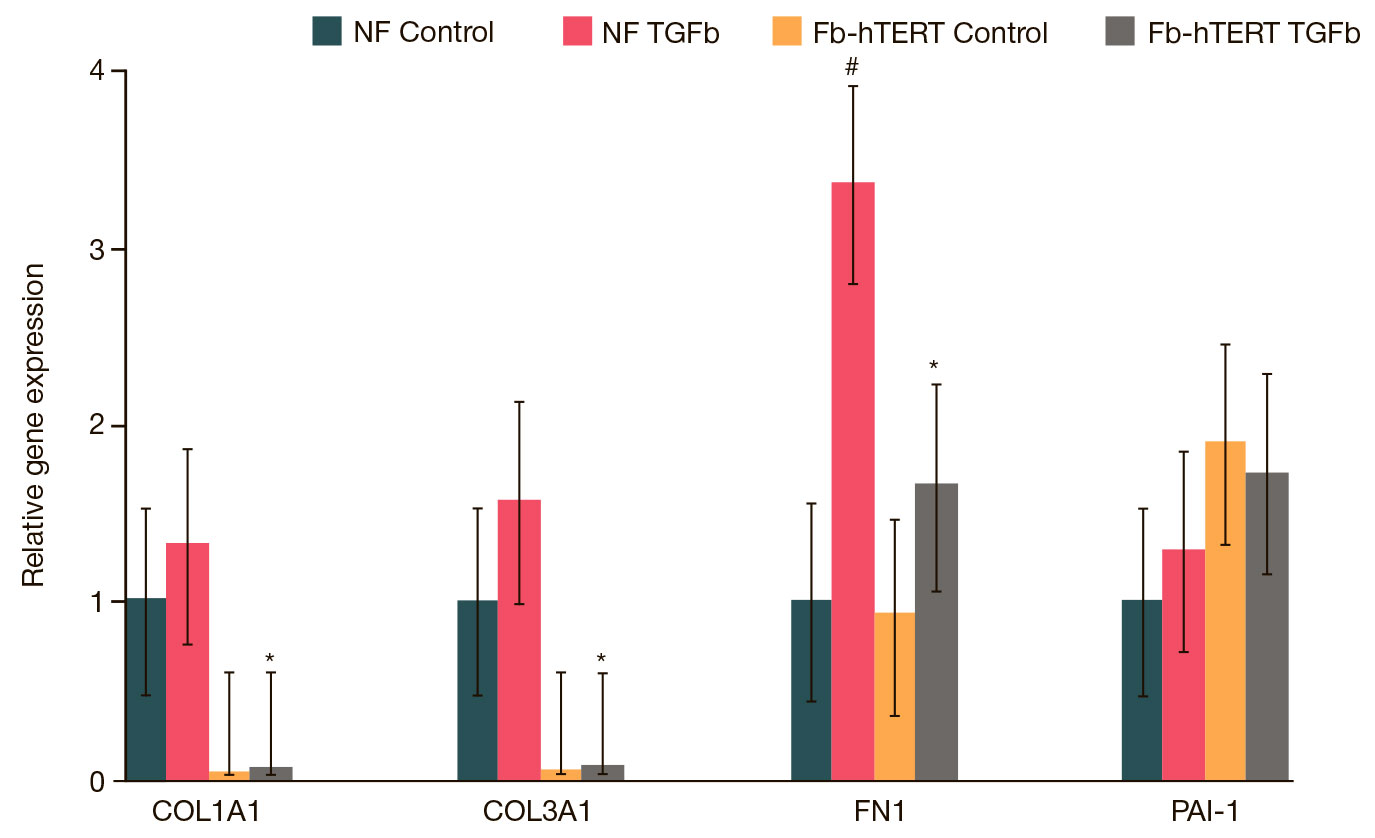
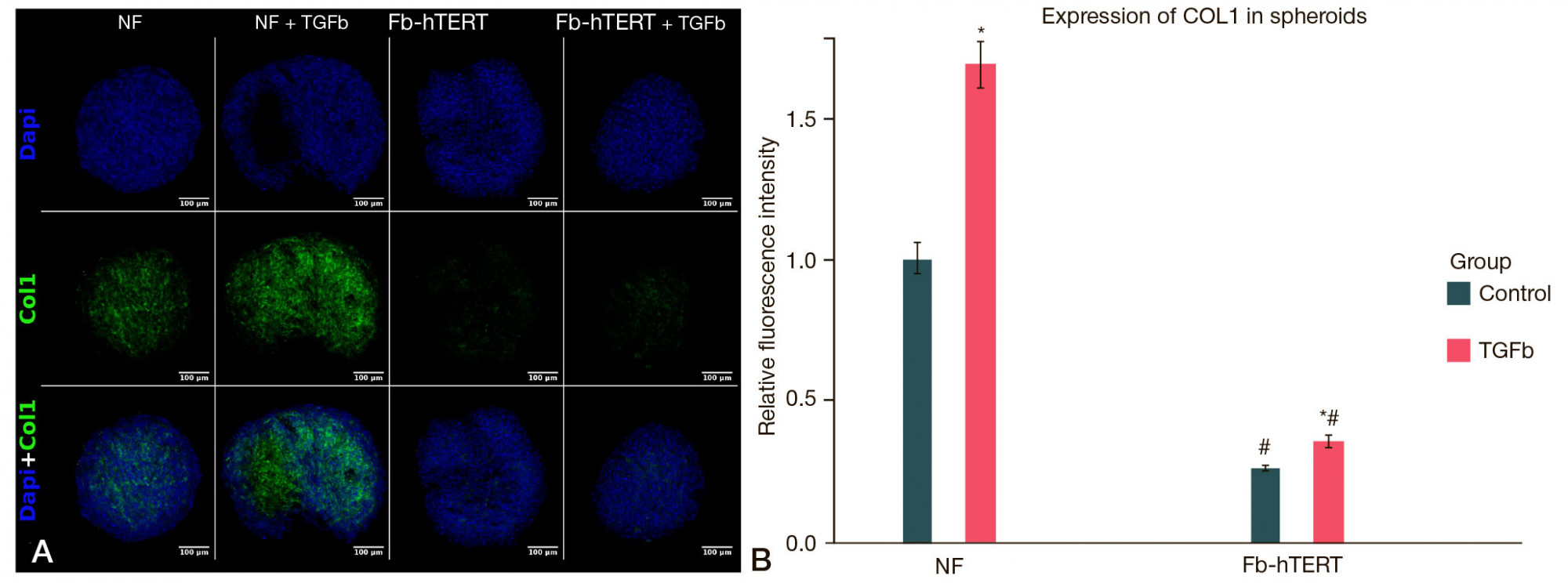
Telomerized fibroblasts as a candidate 3D in vitro model of pathological hypertrophic scars
Orekhovich Research Institute of Biomedical Chemistry, Moscow, Russia
Correspondence should be addressed: Valerian S. Shadrin
Pogodinskaya, 10, str. 8, Moscow, 119121; moc.liamg@nirdahsnairelav
Funding: this research was supported by the Russian Ministry of Science and Higher Education and was conducted under the Federal Targeted Program on Research and Development in Priority Fields of Science and Technology for 2014–2020 (Agreement 05.604.21.0219, Project ID RFMEFI60419X0219).
Author contribution: Luzgina NG, Rusanov AL conceived the study and proposed its design; Shadrin VS, Kozhin PM, Shoshina OO, Luzgina NG, Rusanov AL analyzed the literature, analyzed and interpreted the experimental data and wrote the manuscript; Shadrin VS, Kozhin PM planned and conducted the experiment; Shadrin VS wrote the manuscript.