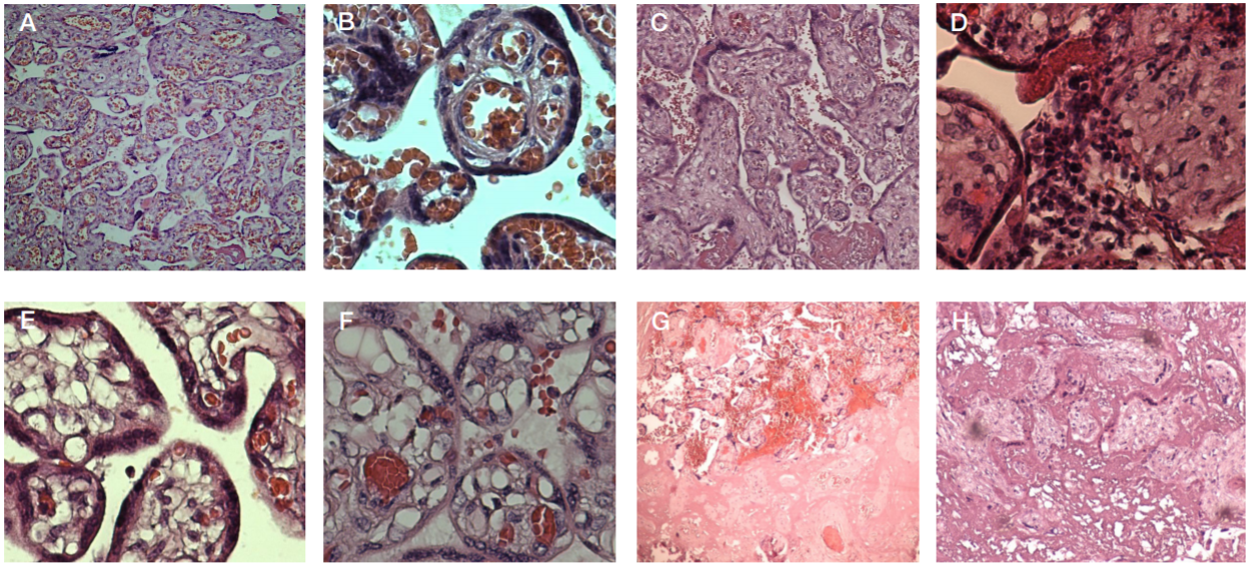
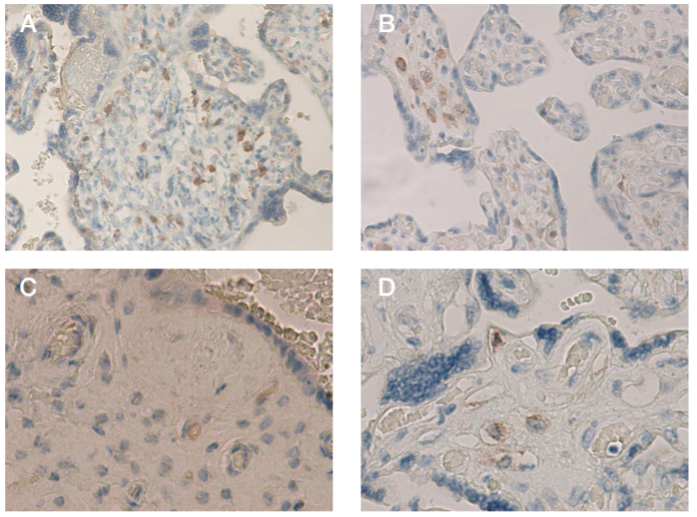
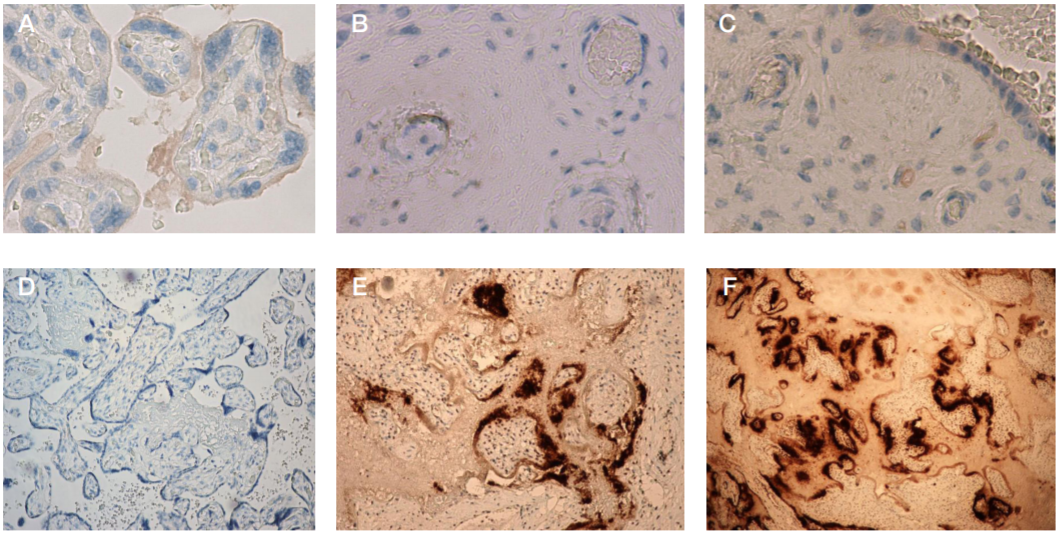
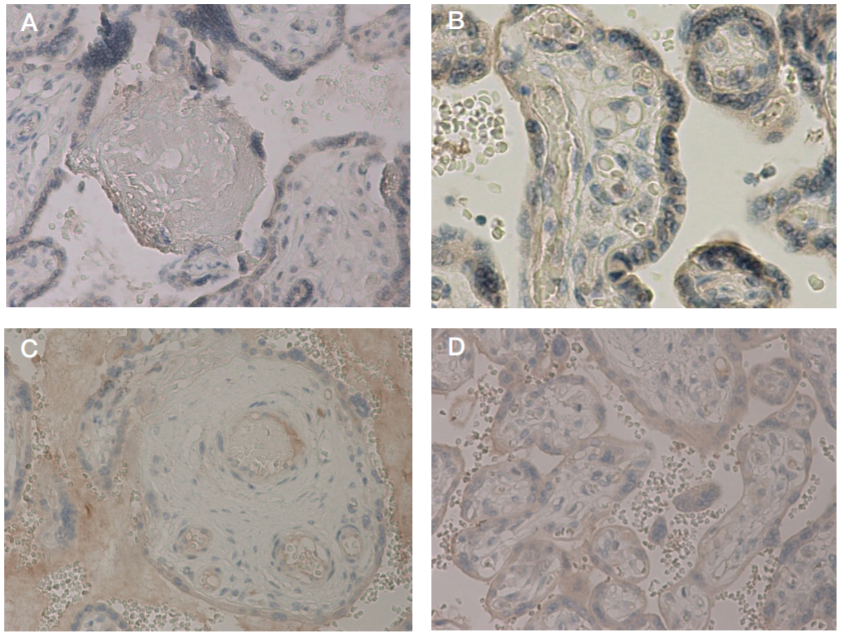
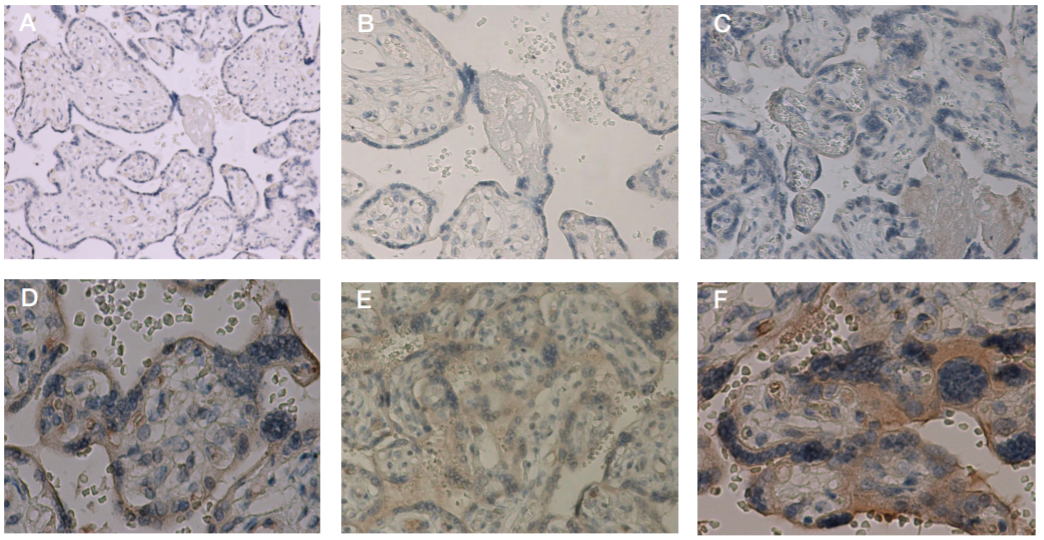
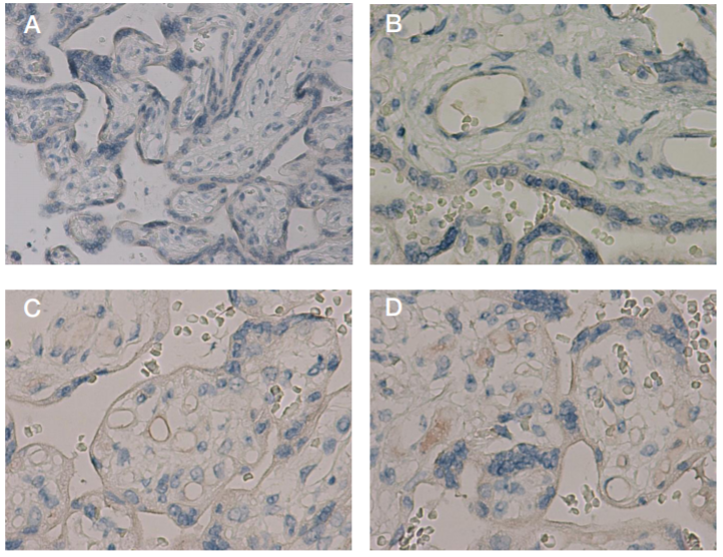
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (CC BY).
ORIGINAL RESEARCH
The impact of the novel coronavirus infection COVID-19 on the mother-placenta-fetus system
Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Moscow, Russia
Correspondence should be addressed: Natalia A. Lomova
Oparina, 4, Moscow, 117997; ur.xednay@avomol-ahsatan
Funding: the study was supported by RFBR grant № 20-04-60093.
Acknowlegements: here we would like to thank Sinitsina VA, medical laboratory assistant of the 2nd Anatomical Pathology Department of the Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, and Bugrova AV, senior research scientist of the Laboratory for Proteomics and Metabolomics of Human Reproduction of the Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology.
Author contribution: Nizyaeva NV — pathomorphological study and IHC analysis, systematic analysis, manuscript writing; Lomova NA — analysis of clinical data, systematic analysis, manuscript writing; Dolgopolova EL — collection and preparation of biological matrix samples in the red zone, statistical analysis of the results; Petrova UL — collection and preparation of biological matrix samples in the red zone; Karapetyan ТE — analysis of clinical data; Shmakov RG — analysis of clinical data in the red zone, systematic analysis, manuscript editing; Frankevich VE — preparation of the study, systematic analysis.
Compliance with ethical standards: all patients submitted the informed consent to participate in the study; the study met the requirements of the Declaration of Helsinki, International Conference on Harmonization (ICF), Good Clinical Practice (GCP), and Federal Law No. 323-FZ “On the Basics of Protecting Citizens' Health in the Russian Federation” of November 21, 2011.