This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (CC BY).
ORIGINAL RESEARCH
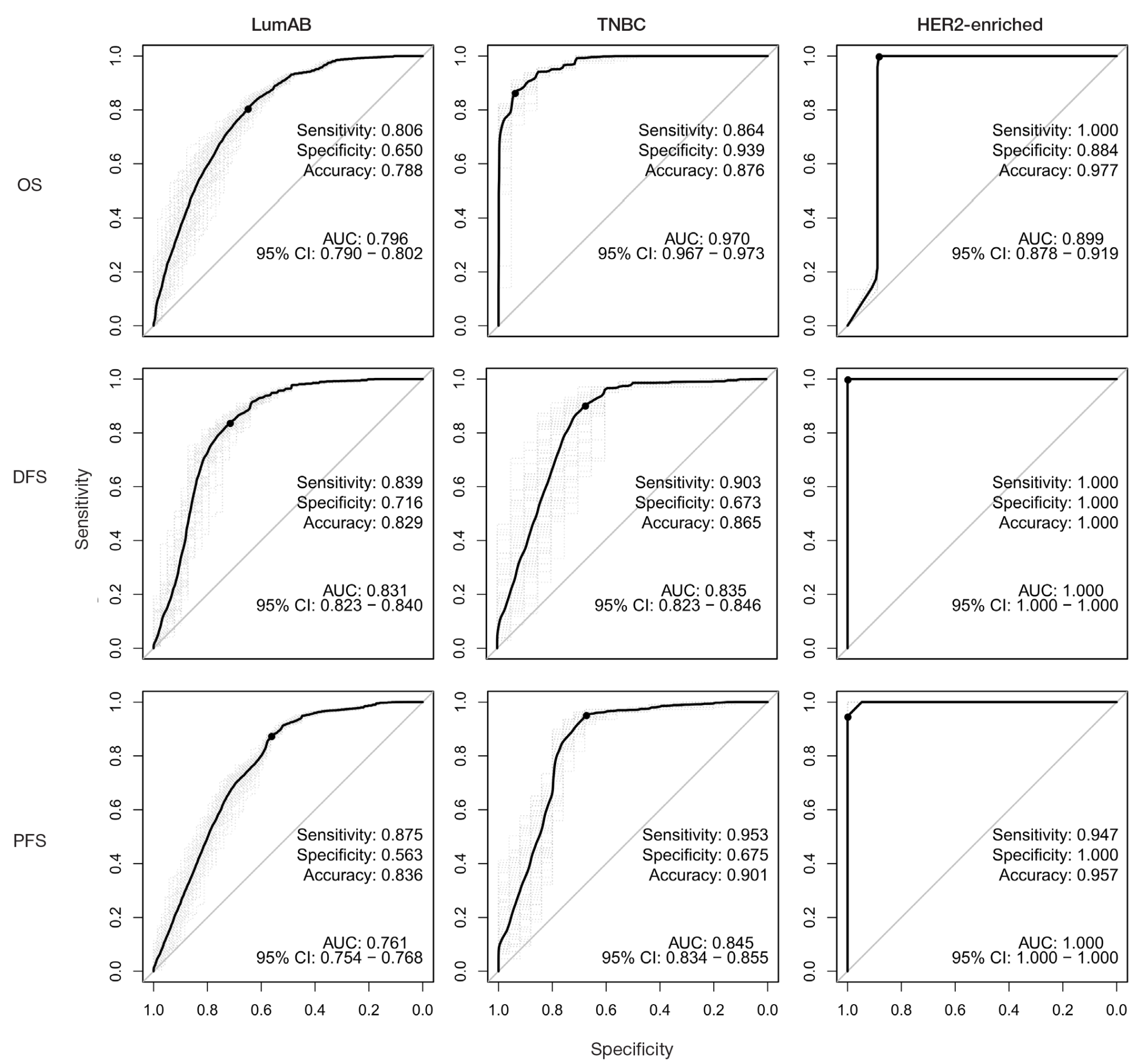
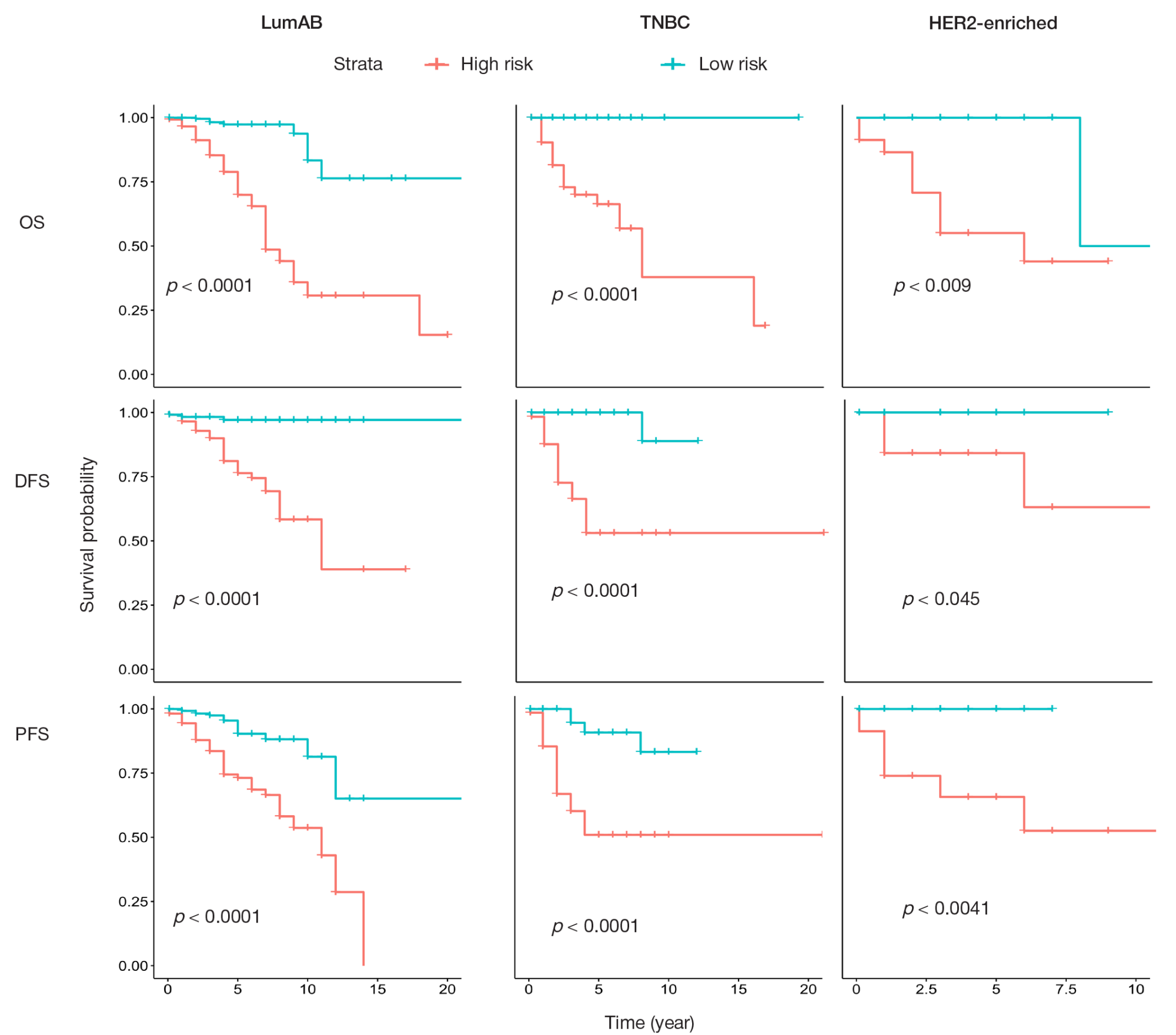
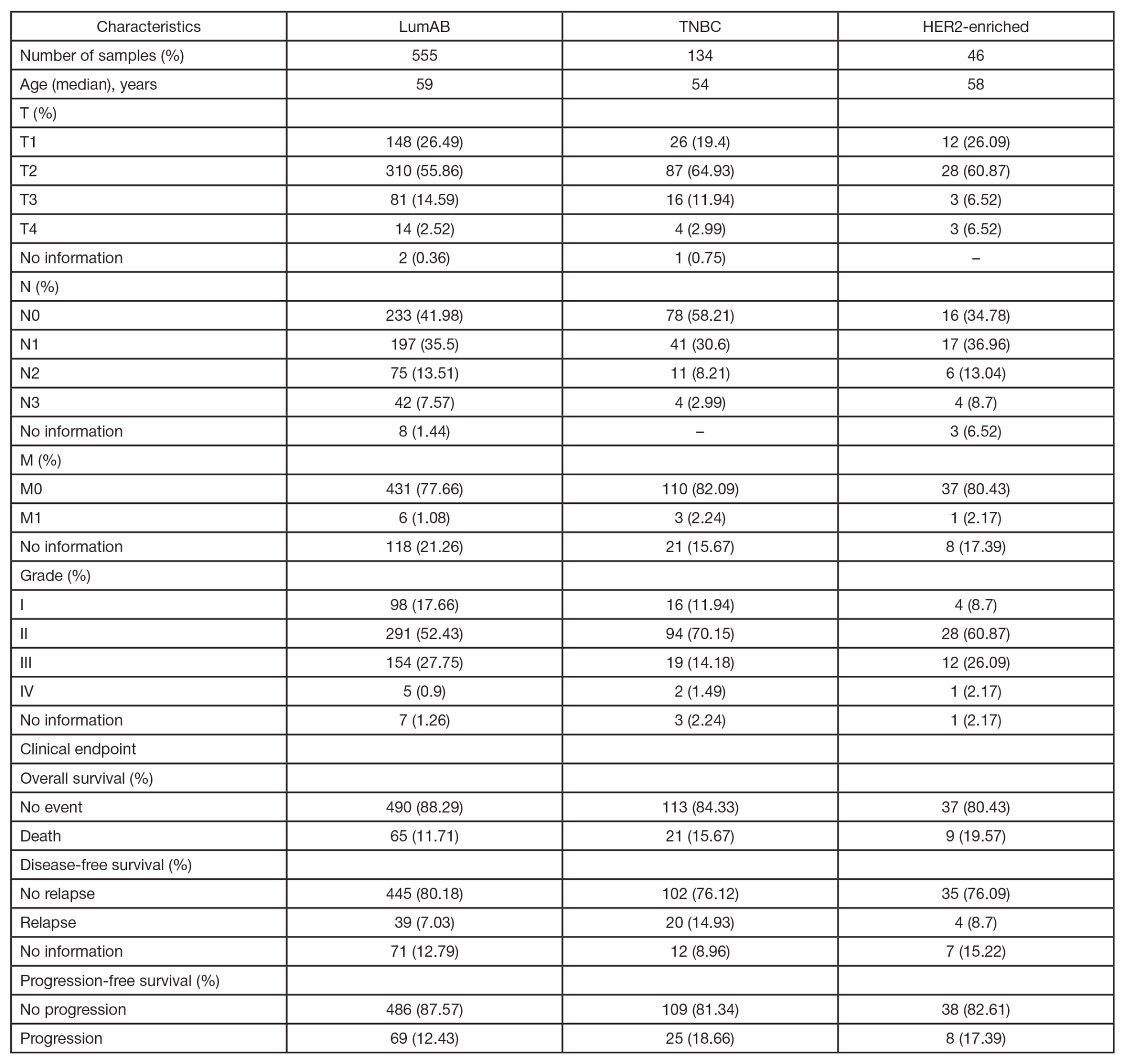
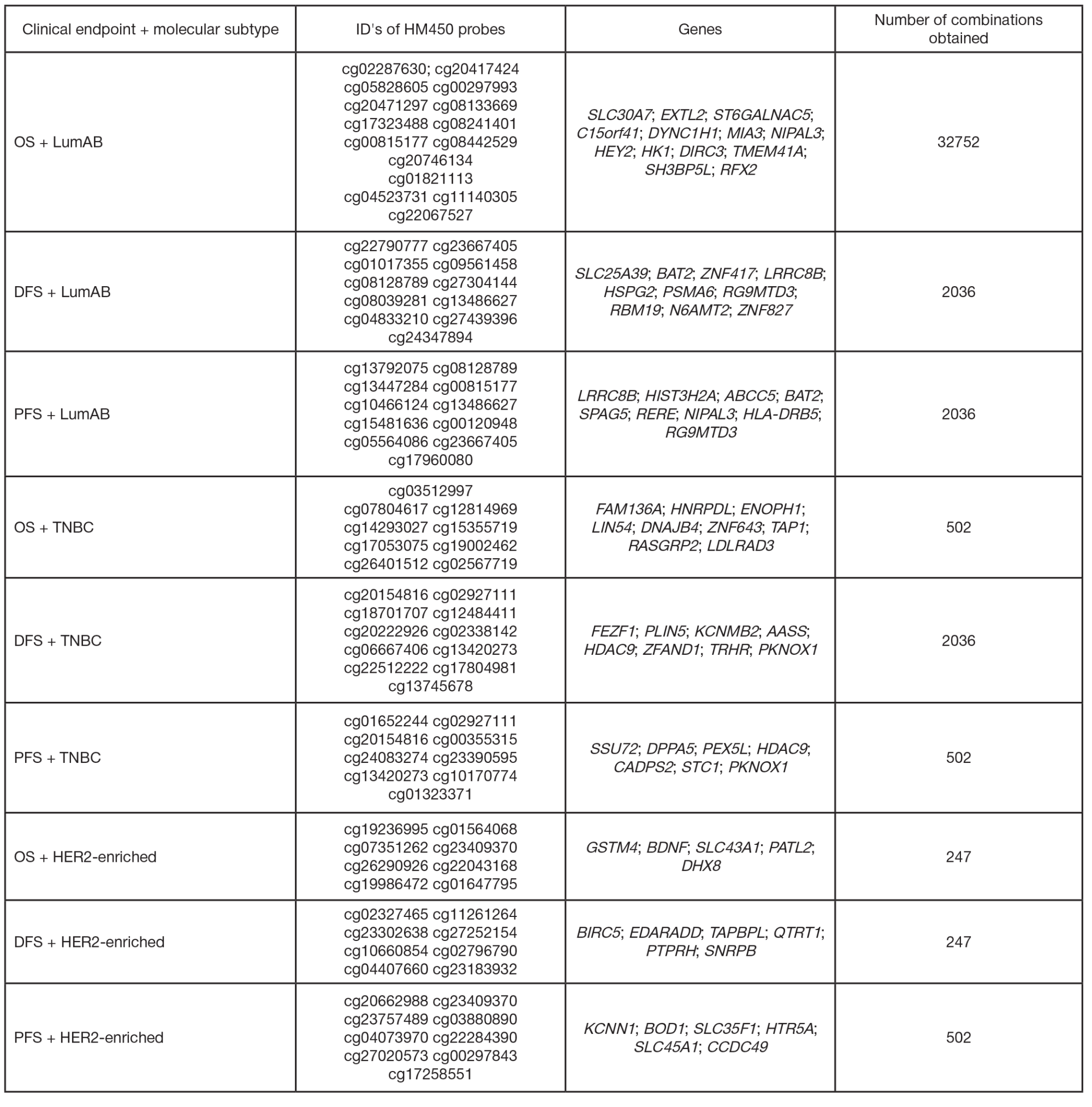
Identification of prognostically significant DNA methylation signatures in patients with various breast cancer types
1 Research Centre for Medical Genetics, Moscow, Russia
2 Sechenov First Moscow State Medical University (Sechenov University), Moscow, Russia
3 Pirogov Russian National Research Medical University, Moscow, Russia
Correspondence should be addressed: Alexey I. Kalinkin
Moskvorechye, 1, Moscow, 115522; ur.xednay@2akiexela
Funding: the study was supported by the Ministry of Science and Higher Education of the Russian Federation within the framework of the Federal Scientific and Technical Program for the Development of Genetic Technologies in 2019–2027 (agreement № 075-15-2021-1073).
Author contribution: Kalinkin AI — study design, data acquisition, analysis and interpretation, manuscript writing; Sigin VO — manuscript writing; Nemtsova MV — study concept and design; Strelnikov VV — study concept and design, scientific editing.