This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (CC BY).
ORIGINAL RESEARCH
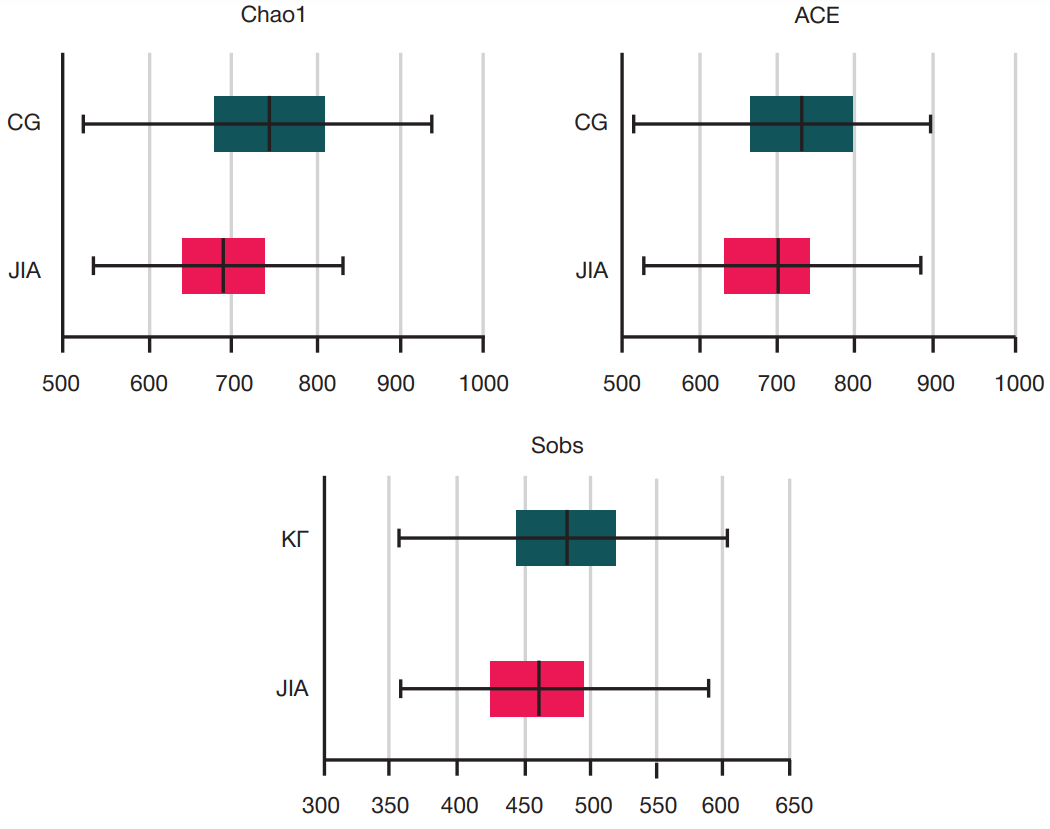
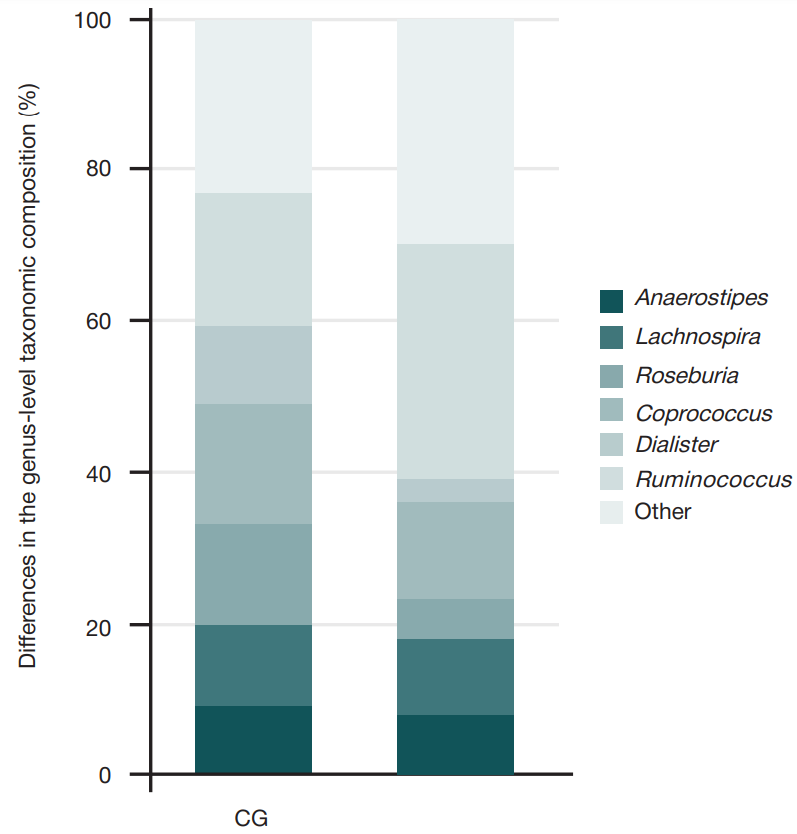
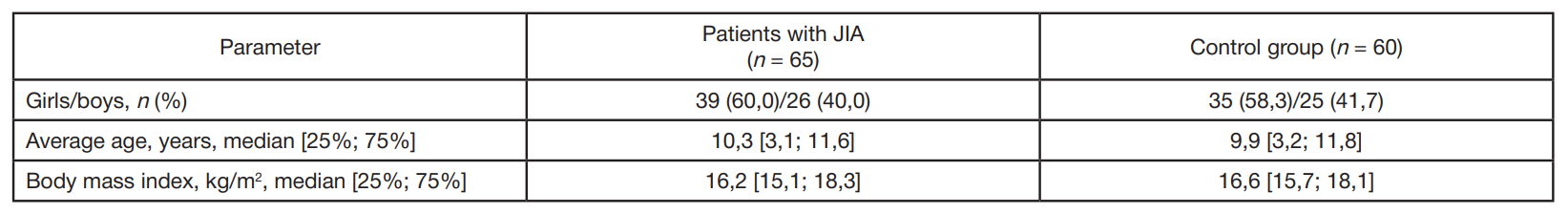
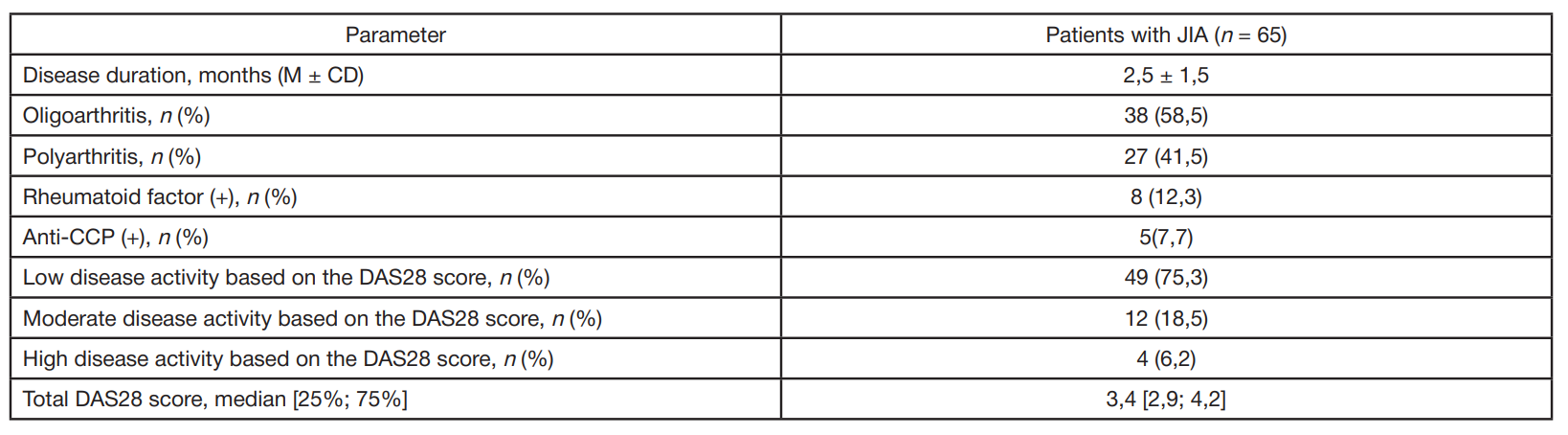
Gut microbiota alterations in patients with juvenile idiopathic arthritis
Georgievsky Medical Academy, Vernadsky Crimean Federal University, Simferopol, Russia
Correspondence should be addressed: Lesya N. Gumenyuk
Lenina bulvar, 5/7, Simferopol, 295006, Republic of Crimea; ur.liam@kuynemyg_aysel
Author contribution: Porosyuk MV, Klementiev DD — data acquisition, analysis, and interpretation; Gumenyuk LN — study concept and design; Hodov NA, Esatova ES, Sereda EV — statistical data processing; Chetveruhina-Malova KS, Sarchuk EV, Ivanov SV — manuscript writing
Compliance with ethical standards: the study was approved by the Ethics Committee of the S.I. Georgievsky Medical Academy, V. I. Vernadsky Crimean Federal University (protocol № 10 of 16 November 2020), planned and conducted in accordance with the Declaration of Helsinki. The infirmed consent was submitted by all the subjects enrolled.