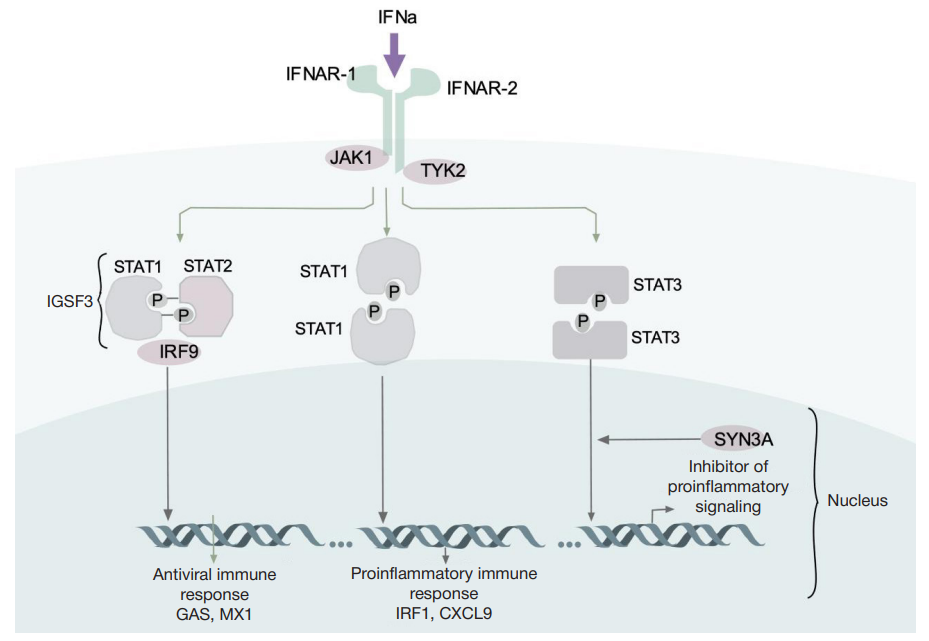
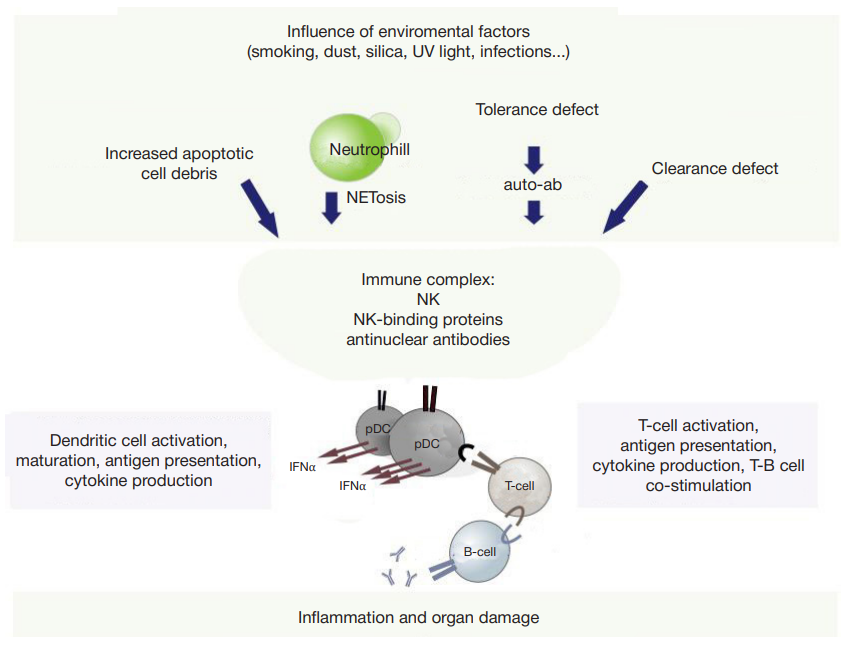
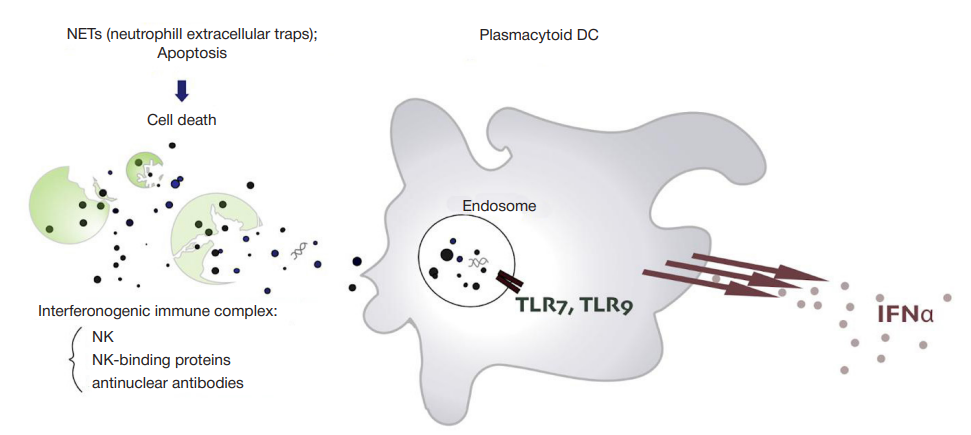
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (CC BY).
OPINION
Interferon signature in the development of SLE: molecular mechanisms, approaches to diagnosis and treatment
1 Shemyakin and Ovchinnikov Institute of Bioorganic Chemistry Russian Academy of Sciences, Moscow, Russia
2 Pirogov Russian National Research Medical University, Moscow, Russia
3 LLC MiLaboratory, Moscow, Russia
4 Department of Rheumatology, City Clinical Hospital No. 52 of the Department of Health, Moscow, Russia
Correspondence should be addressed: Olga V. Britanova
Miklouho-Maklaya, s. 16/10, 117997, Moscow, Russia; moc.liamg@natirblo
Financing: the study was supported by a grant from the Moscow Government (NIP No. 2412-63/22-1 dated May 13, 2022), sponsored by the Moscow Center for Innovative Technologies in Healthcare.
Author contribution: Myshkin MYu — literature analysis; Mutovina ZYu, Shagina IA — data collection in the field of rheumatology and medicine; Chudakov DM — concept, Turchaninova MA — analysis and interpretation of scientific data; Ryazantseva EV, Kazhdan MA — manuscript proofreading, Britanova OV, Nakonechnaya TO — literature analysis and manuscript preparation.