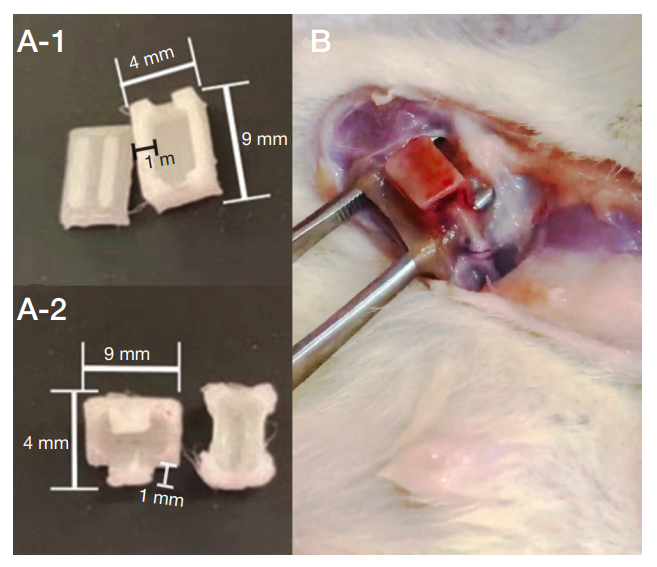
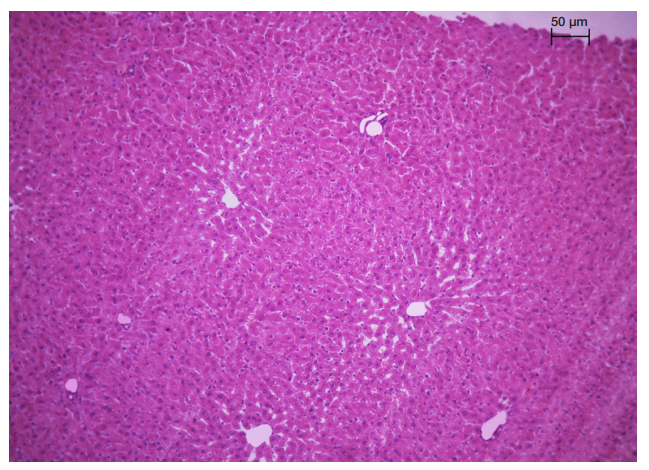
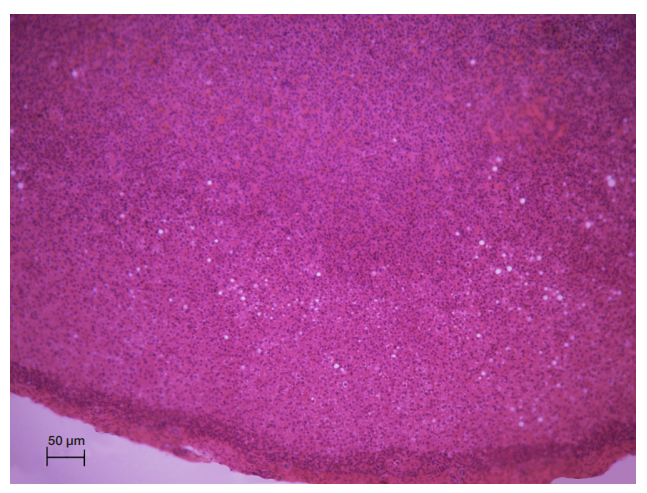
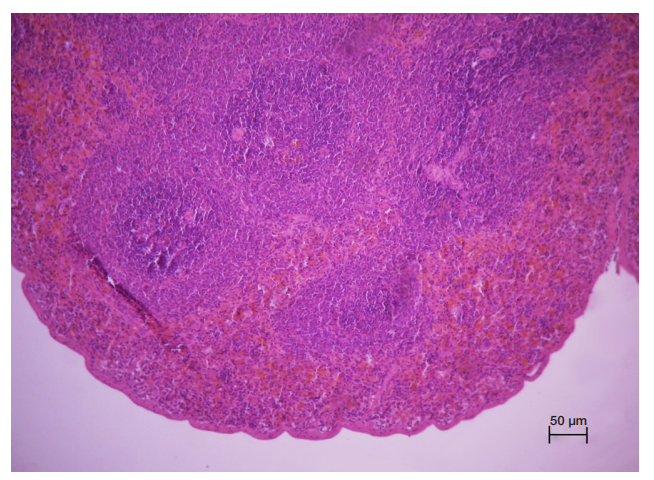
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (CC BY).
ORIGINAL RESEARCH
Estimation of diffusion chamber biocompatibility in the experimental model of implantation in the neurovascular bundle
Siberian State Medical University, Tomsk, Russia
Correspondence should be addressed: Ekaterina A. Marzol
Kartashova, 29b, kv. 78, Tomsk, 634 041; ur.liam@3084aytaK
Funding: the study was supported by the RSF (research project No. 23-25-00346).
Author contribution: Marzol EA, Dvornichenko MV — developing concept and design; Marzol EA, Aparshev NA, Mitryaikin NS — data analysis and interpretation; Marzol EA, Mitryaikin NS, Dvornichenko MV — substantiation of manuscript or verification of critical intellectual content; Dvornichenko MV — final approval of manuscript before publishing.
Compliance with ethical standards: the study was approved by the Ethics Committee of the Siberian State Medical University (protocol No. CDI-005 dated 5 February 2022). Animals were handled in accordance with the Directive 2010/63/EU of the European Parliament and the Council on the protection of animals used for scientific purposes dated 22 September 2010, rules and regulations of the European Community (86/609EEC), Declaration of Helsinki, and orders of the Ministry of Health of the USSR (No. 742 dated 13 November 1984 and No. 48 dated 23 January 1985).