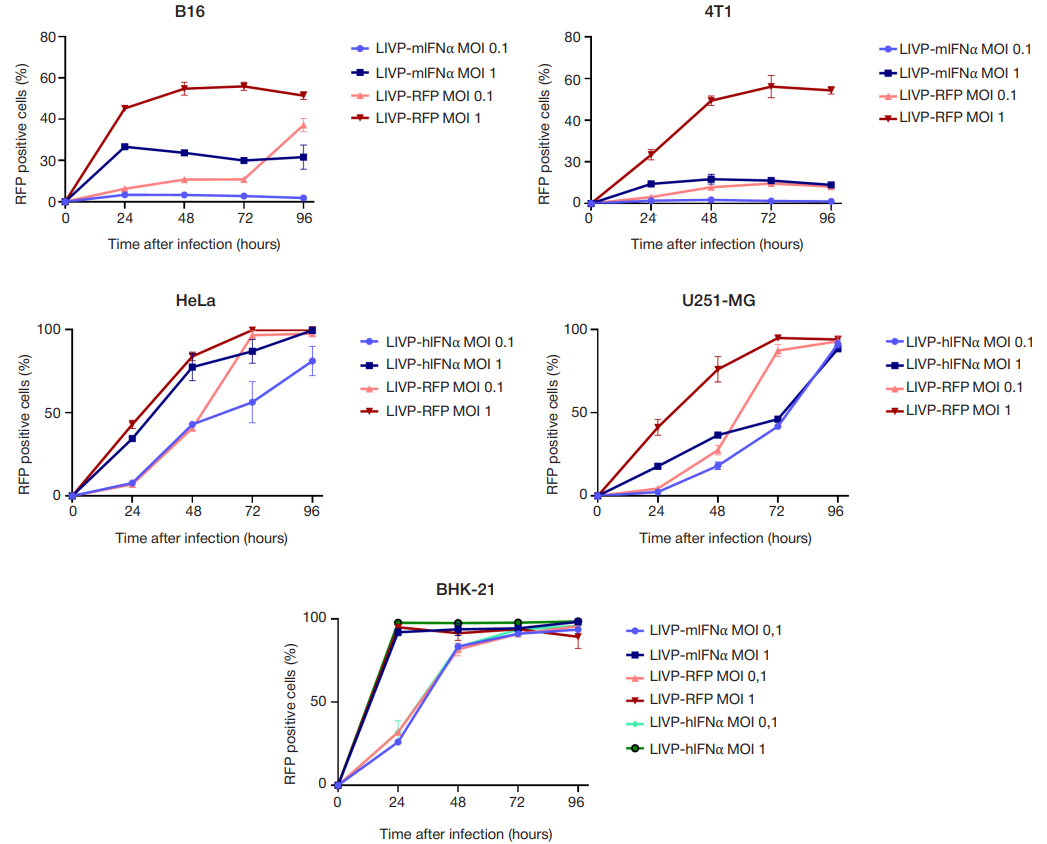
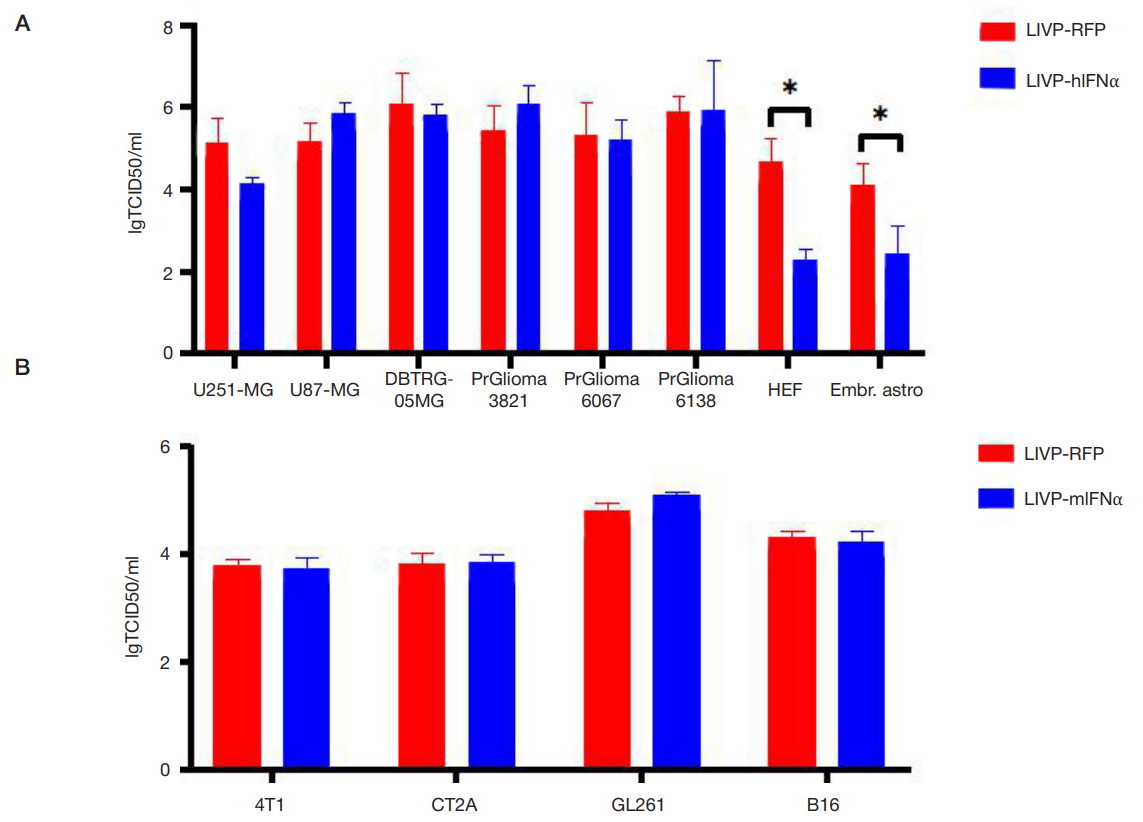
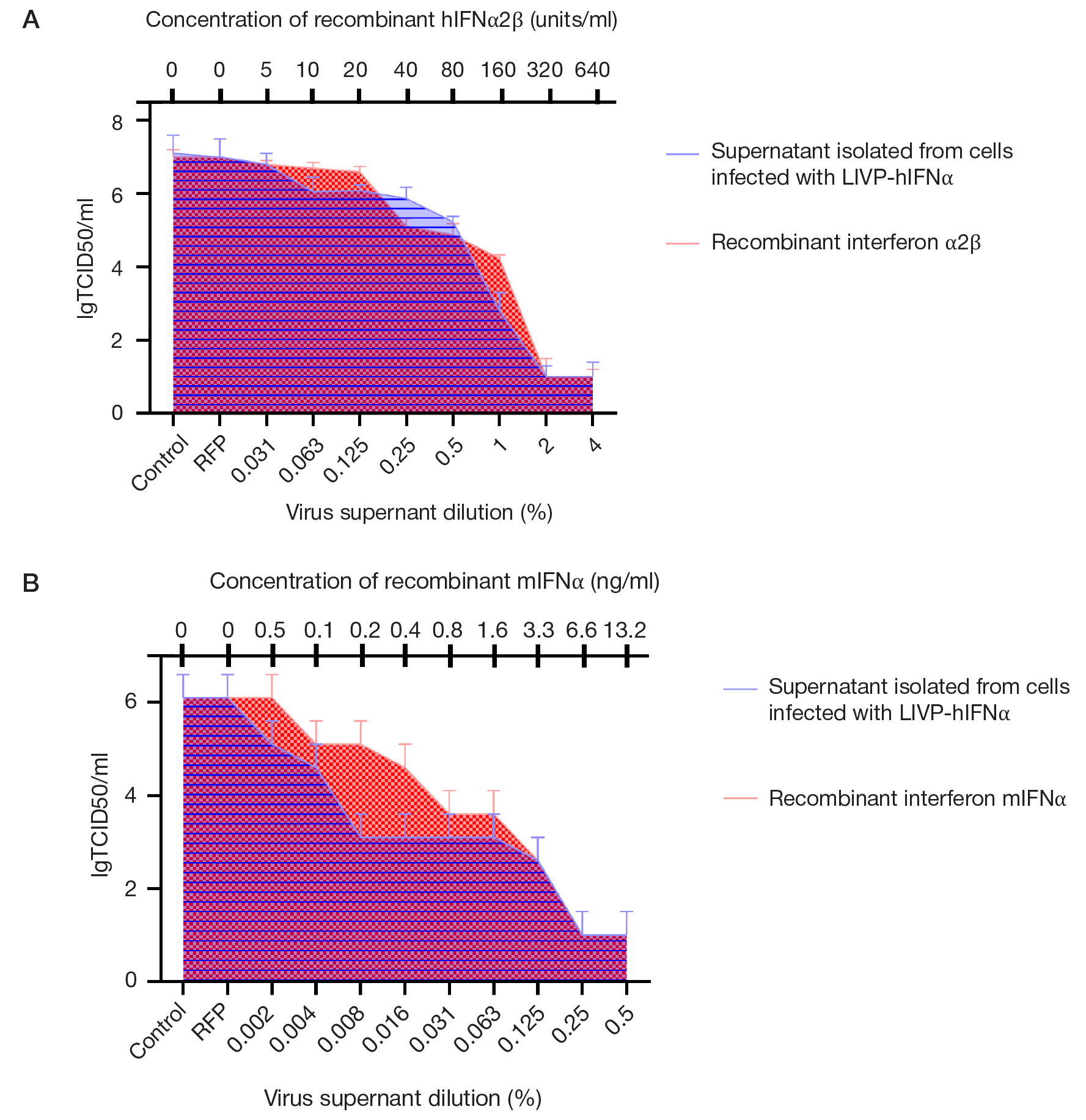
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (CC BY).
ORIGINAL RESEARCH
Interferon type I-expressing recombinant vaccinia virus as a platform for selective immunotherapy of glioblastoma and melanoma
1 Engelhardt Institute of Molecular Biology, Russian Academy of Science, Moscow, Russia
2 Federal Scientific and Clinical Center of Specialized Types of Medical Care and Medical Technologies, Federal Medical Biological Agency of Russia, Moscow, Russia
3 Federal Center of Brain Research and Neurotechnologies of the Federal Medical Biological Agency, Moscow, Russia
4 Research Institute of Pulmonology of the Federal Medical Biological Agency, Moscow, Russia
Correspondence should be addressed: Anastasia V. Lipatova
Vavilova, 32/1, Moscow, 119991, Russia; moc.liamg@vnaavotapil
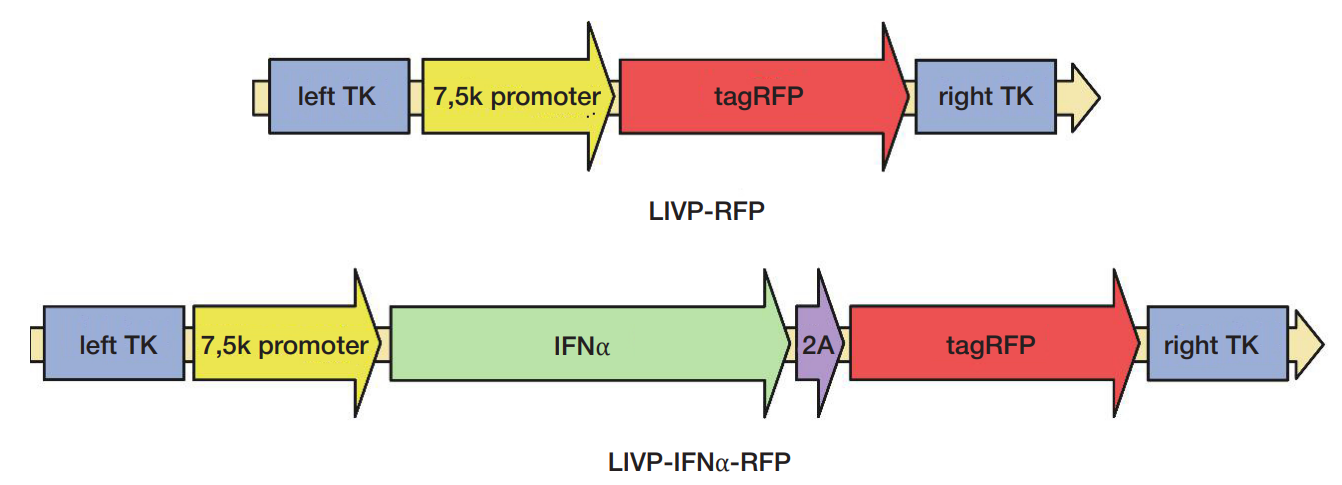
Funding: the design of recombinant vaccinia virus strains was supported by the Russian Science Foundation grant No. 23-14-00370, and assessment of their properties in in vitro and in vivo models was supported by the Russian Science Foundation grant No. 22-64-00057; histologic and immunohistochemical assessment of tumor tissue was supported by FMBA of Russia.
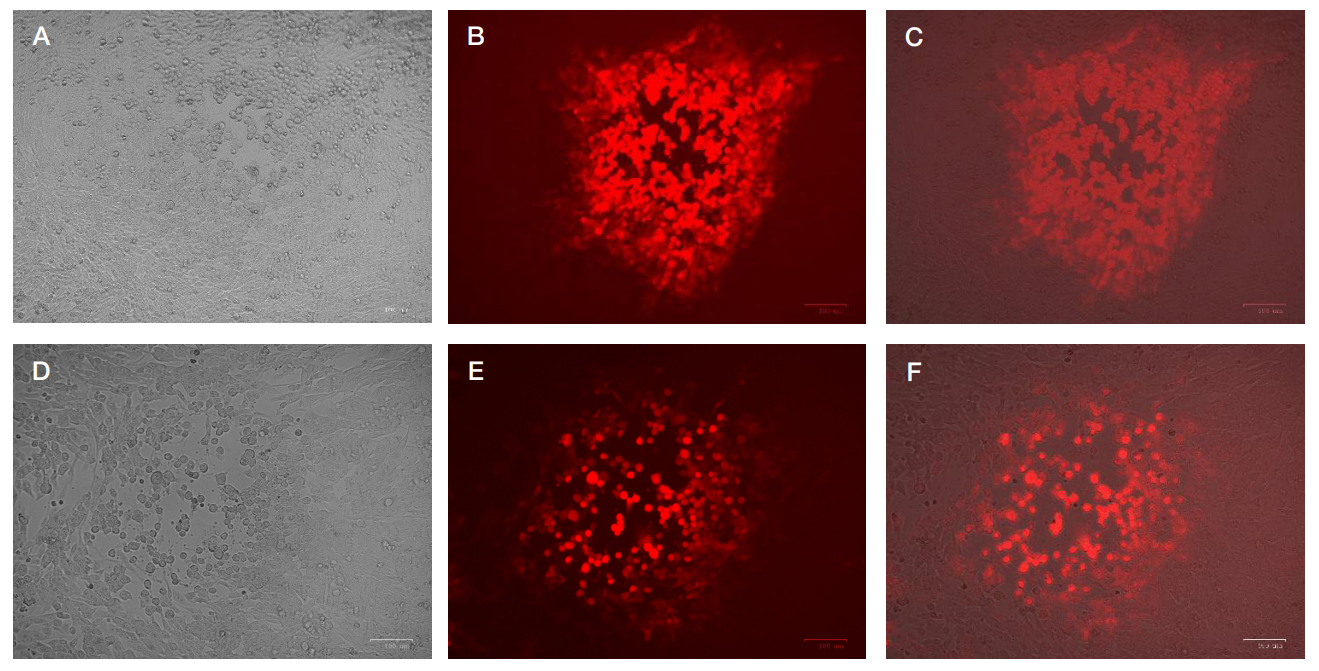
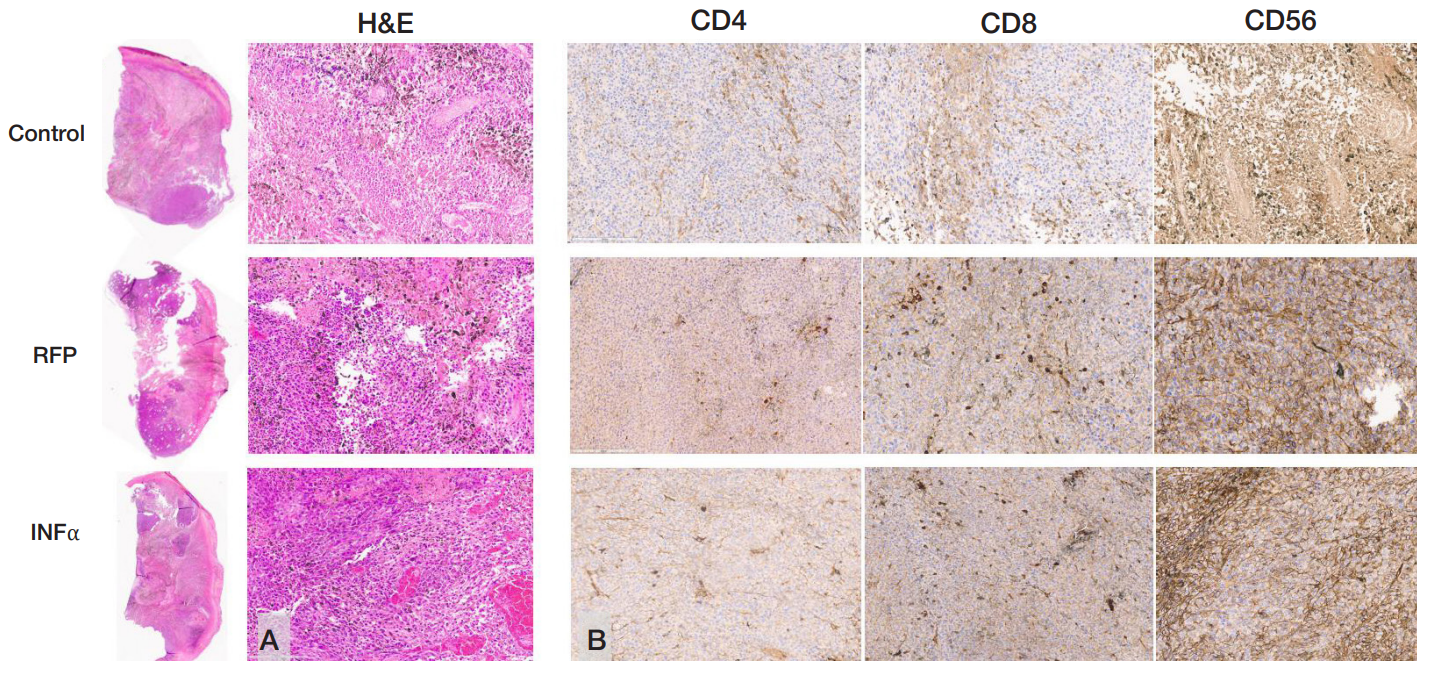
Author contribution: Naberezhnaya ER — implementation of in vitro and in vivo experiments, manuscript writing; Soboleva AV — flow cytometry data acquisition, determining the cell line sensitivity to viruses; Vorobyev PO — designing recombinant viruses, production of preparative amounts of strains; Vadekhina VV — conducting in vivo experiments; Yusubalieva GM — interpretation of in vivo experimental data, manuscript writing; Isaeva IV — histologic and immunohistochemical assessment; Baklaushev VP — microscopy, describing histology and immunohistochemistry data, preparing a drawing, manuscript writing; Chumakov PM — manuiscript editing; Lipatova AV — study concept, general project management.
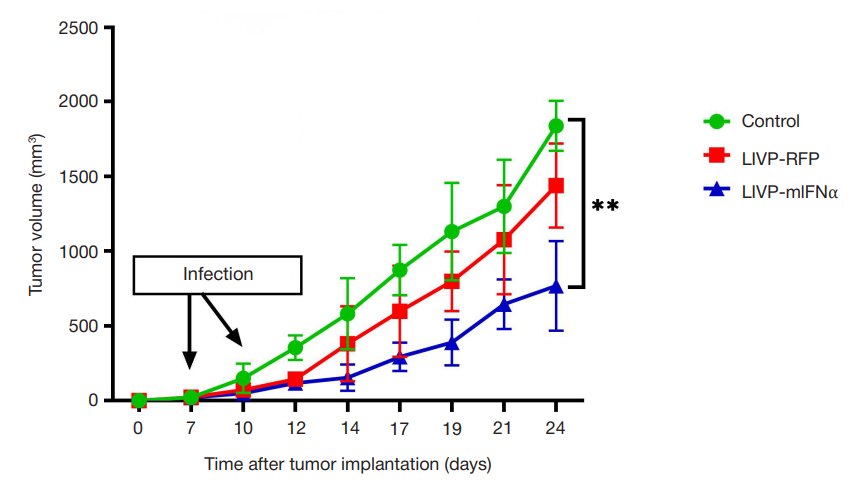
Compliance with ethical standards: the in vivo study was approved by the Ethics Committee of the Federal Scientific and Clinical Center of FMBA of Russia (protocol No. 7 dated 06 September 2022) and conducted in accordance with the the Eurasian Economic Commission Board’s guidelines No. 33 dated 14 November 2023 "On the Guidelines for handling laboratory (experimental) animals when conducting preclinical (non-clinical) studies". The number of animals per group was minimized; the subcutaneous tumor size in the groups did not exceed 2000 mm3. In vitro experiments involved the commercially available animal and human cell lines.