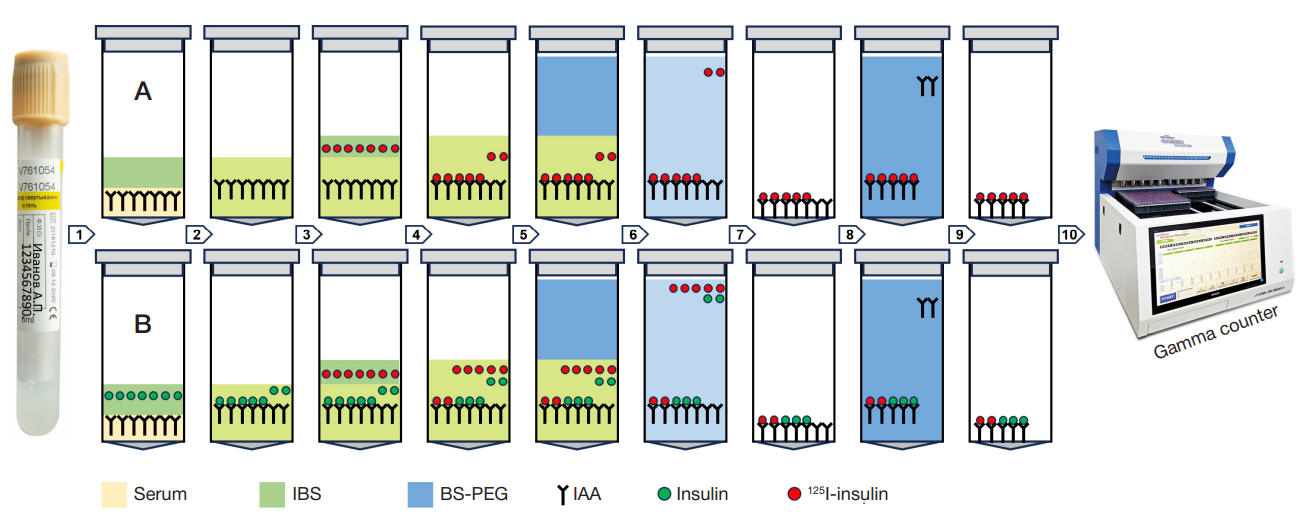
Fig. 1. RIA IAA procedure 1. Serum samples (75 µL) were poured into two series of 1.7 mL Eppendorf conical tubes (Costar 3207, Corning). A total of 75 µL of the 0.23М incubation buffer solution (IBS), pH 7.4 with the following composition were added to each A series tube: NaH2PO4 (Sigma-Aldrich, REF S-0751) 0.014М; NaH2PO4 (Panreac, REF 141677) 0.067М; NaCl (Sigma-Aldrich, REF S-9625) 0.15М; bovine serum albumin (CDH, REF TC1546) 0.05%; Twin-20 (Panreac, REF 162312) 0.05%. A total of 75 µL of the IBS with the recombinant human insulin (Insulin Reference Standard, Eli Lilly, USA) at a concentration of 9 × 10–3 U/mL were added to each B series tube. 2. Test tubes of both series were vortexed with a vortex mixer and incubated for 30 min on the на ELMI-ST3 orbital shaker (Elmi, Latvia) with the platform rotation speed 250 rpm at room temperature. IAA bound to insulin during incubation. 3. A total of 100 µL of IBS with the recombinant human insulin labeled with the 125I (125I-insulin) at a concentration of 7.5 × 10–6 U/mL were added to each tube of both series. Furthermore, 100 µL of IBS with the 125I-insulin were added to each of two tubes to calculate total radioactivity. The 125I-insulin preparation was produced in the Institute of Bioorganic Chemistry by monoiodination of insulin based on tyrosine A14 using chloramine-T as an oxidizing agent, purified by gel filtration of the column with Sephadex G-15. Iodination involved the use of sodium iodide (125I) (ISOTOPE, RF). Finally, we obtained a stabilized 125I-insulin preparation with the following radiochemical charateristics: total activity — 352 kBq, specific activity — 58 TBq/mmol, radiochemical purity — 92%. 4. All the test tubes were sealed and incubated for 7 days in a refrigerator at +4 °С. During incubation the serum IAA bound to insulin and 125I-insulin, and the equilibrium between IAA binding with the labeled and non-labeled ligands established. 5. A total of 500 µL of the buffer solution (pH 8.6) containing polyethyleneglycol (BS-PEG) with the following composition added to all tubes, except those for total activity calculation : Tris 0.05М (Sigma-Aldrich REF 7-9 Tris base T13,78); PEG-8000 (Polyethylenglycol 8000 BioChemica AppliChem REF A2204.0500) 14%. BS-PEG was previously cooled to 0 °С. 6, 7. The tubes were vortexed with a vortex mixer and centriguged in the Beckman G-2-21 centrifuge at 2000 g for 30 min at +4 °С. The supernatant was removed with an aspirator. As a result, a precipitate was obtained containing the IAA complexes with the labeled and unlabeled insulin, as well as IAA that did not bind to insulin. 8, 9. A total of 1000 µL of BS-PEG with the 11% PEG-8000 previously cooled to 0 °С were added to all tubes, except those for TR calculation. The tubes were vortexed with a vortex mixer and centriguged in the Beckman G-2-21 centrifuge at 2000 g for 30 min at +4 °С. The supernatant was removed with an aspirator. As a result, a precipitate was obtained containing the IAA complexes with the labeled and unlabeled insulin. 10. Radioactivity was measured in all the tubes (including those for total activity calculation) with the Wizard γ-spectrometer (PerkinElmer, USA) at a measuring time of 1 min
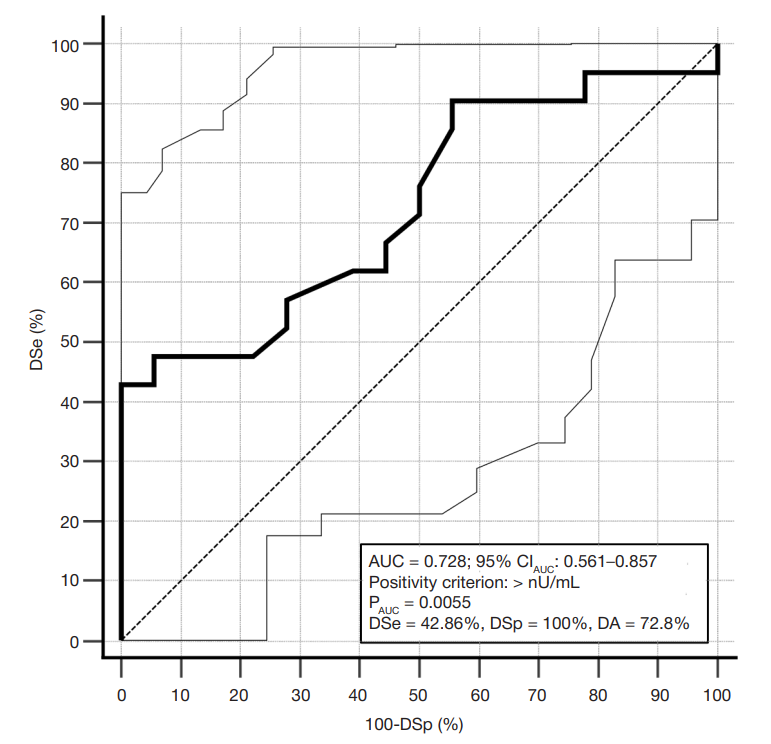
Fig. 2. Receiver Operating Curve of the IAA test. Dashed curve — the line bounding the AUC of 0.5. Solid curve — the test receiver operating characteristic curve. Dotted curves — borders of the 95% confidence interval (95%CIAUC) for the operating characteristic curve. PAUC — probability of significance of the null hypothesis about the lack of difference between AUC 0.5 and AUC of the test
Table 1. Operational parameters of various IAA tests based on the IASP data [7]
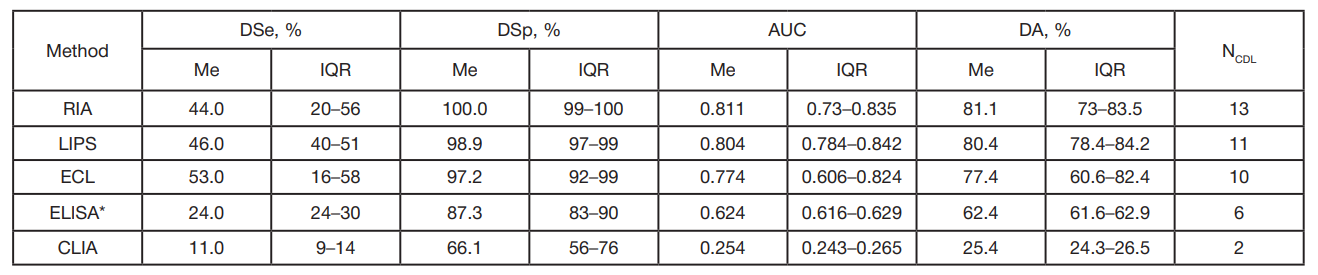
Note: AUC — area under receiver operating curve; NLAB — number of participating labs; Me — median; IQR — interquartile range.* — all labs used home-made test systems (commercially available systems were not used).
Table 2. Results of measuring CIAA in serum samples
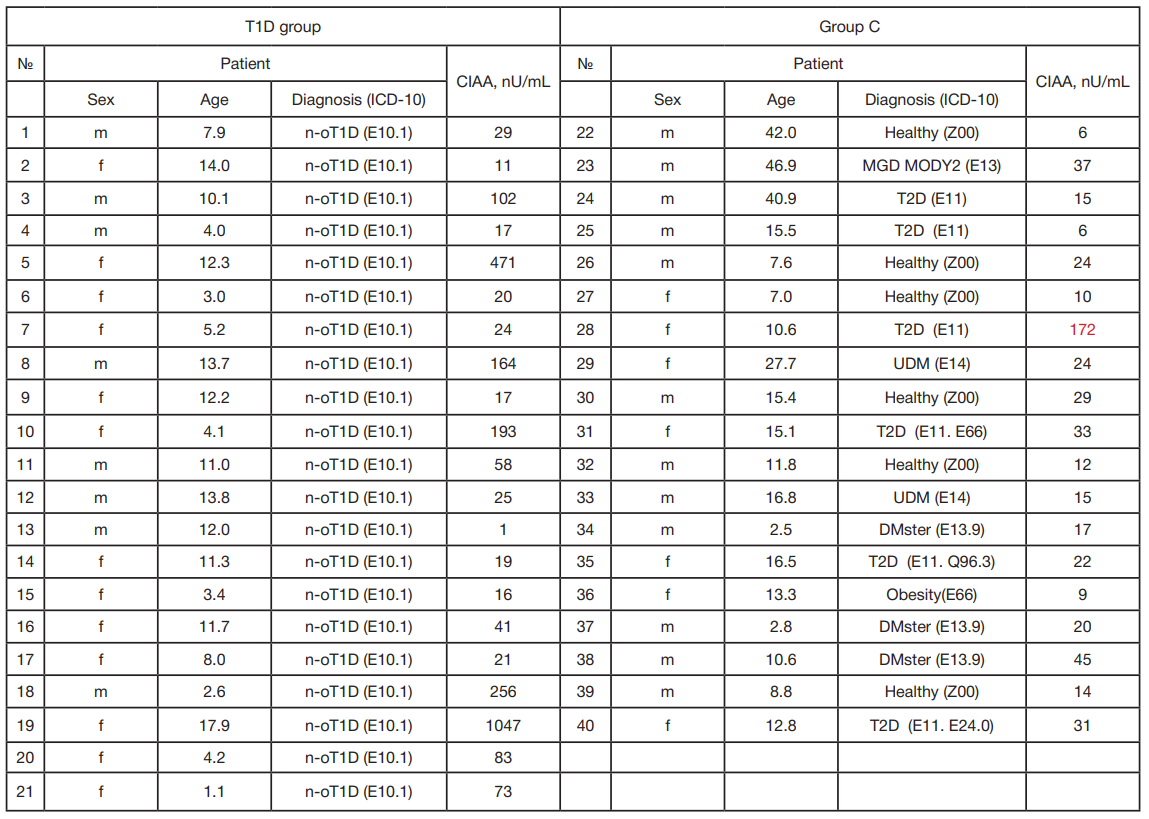
Note: № — ordinal number of the serum sample; NOT1D — new-onset type 1 diabetes mellitus; MGD MODY2 — monogenic diabetes mellitus, MODY2 variant (mutation in the hexokinase gene); T2D — type 2 diabetes mellitus; UDM — diabetes mellitus unspecified; DMster — diabetes mellitus caused by taking glucocorticosteroids. The result classified as an outlier is highlighted in red





