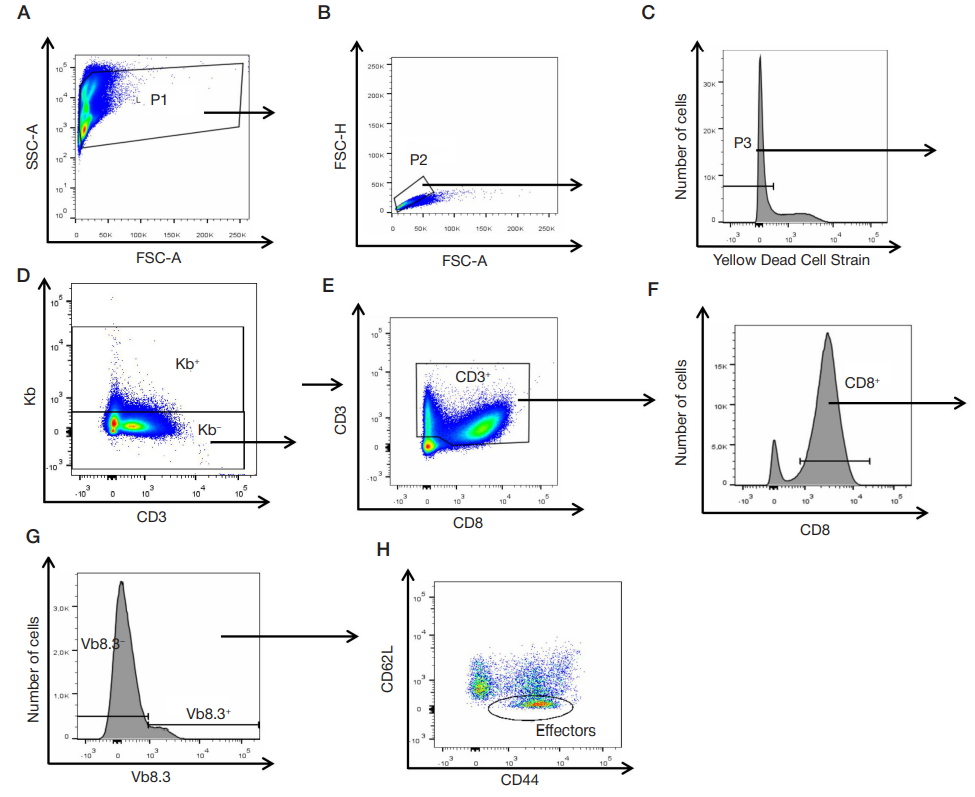
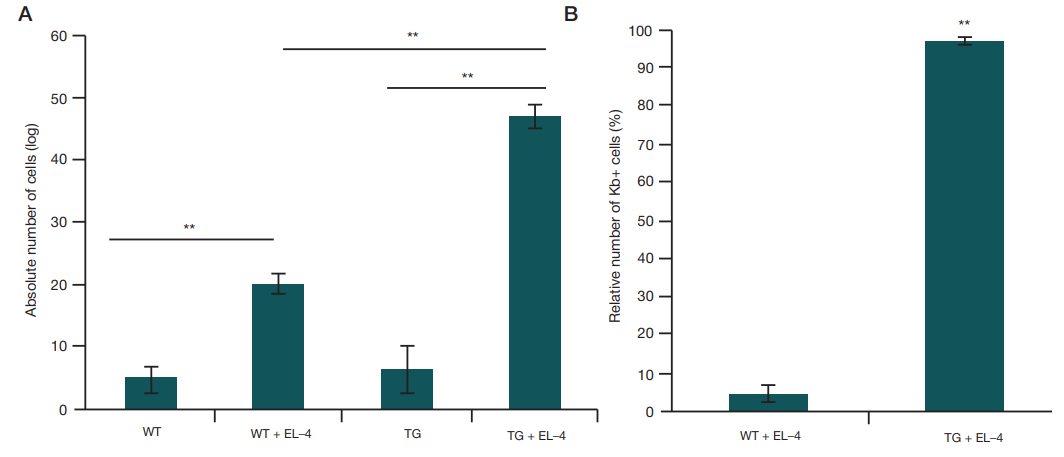
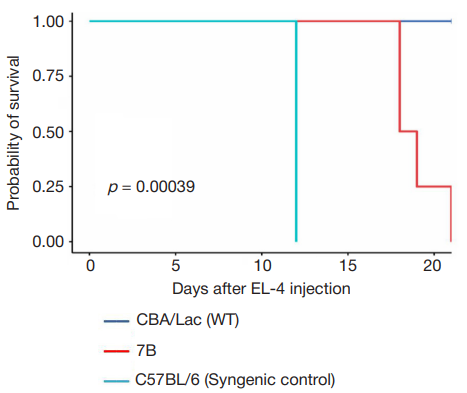
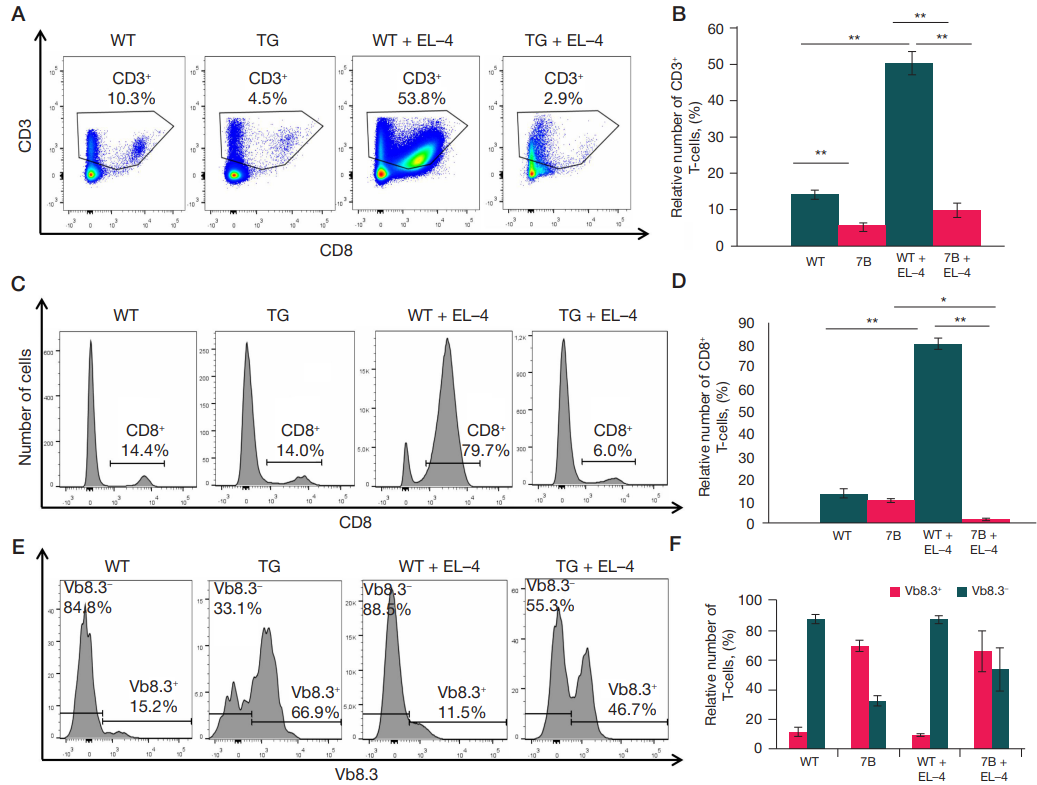
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (CC BY).
ORIGINAL RESEARCH
Features of the immune response to tumor alloantigens in the context of decreased clonal diversity of T cells
Blokhin National Medical Research Center of Oncology, Ministry of Health of the Russian Federation, Moscow, Russia
Correspondence should be addressed: Dmitry B. Kazansky
Kashirskoe shosse, 24, str. 15, Moscow, 115478, Russia; ur.xednay@1yksnazak
Funding: the study was financially supported by the Russian Science Foundation grant No. 22-15-00342-П (https://rscf.ru/project/22-15-00342/).
Author contribution: Korotkova MS — experimental work, analysis of the results; Persiyantseva NA — experimental work, analysis of the results, article editing; Kalinina AA — study planning, analysis and interpretation of the results, analysis of the available literature, article authoring and editing; Khromykh LM — article editing; Kazansky DB — study planning, analysis and interpretation of the results, analysis of the available literature, article editing.
Compliance with ethical standards: the study was approved by the Ethics Committee of the N.N. Blokhin National Research Medical Center of Oncology of the Ministry of Health of the Russian Federation (Minutes No. 3-П10.06.2022 of June 10, 2022) and conducted in strict accordance with the provisions of Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes.