This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (CC BY).
ORIGINAL RESEARCH
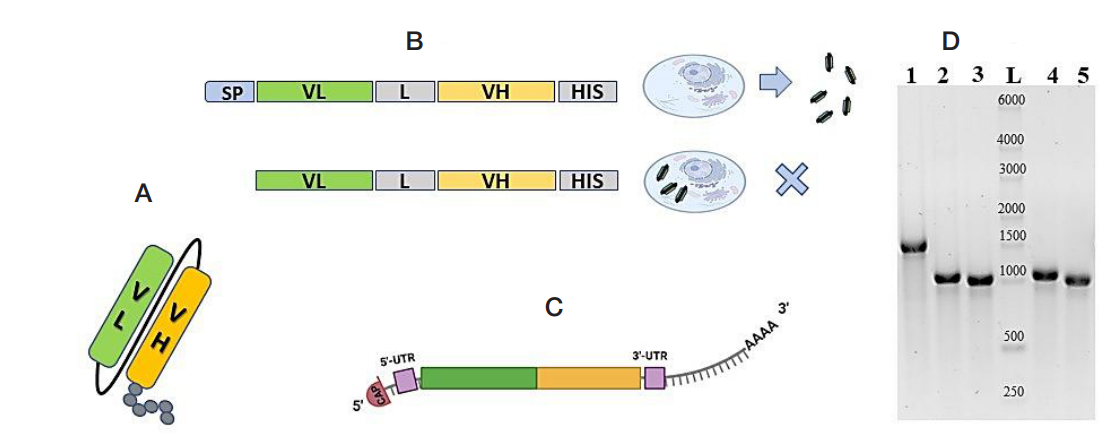
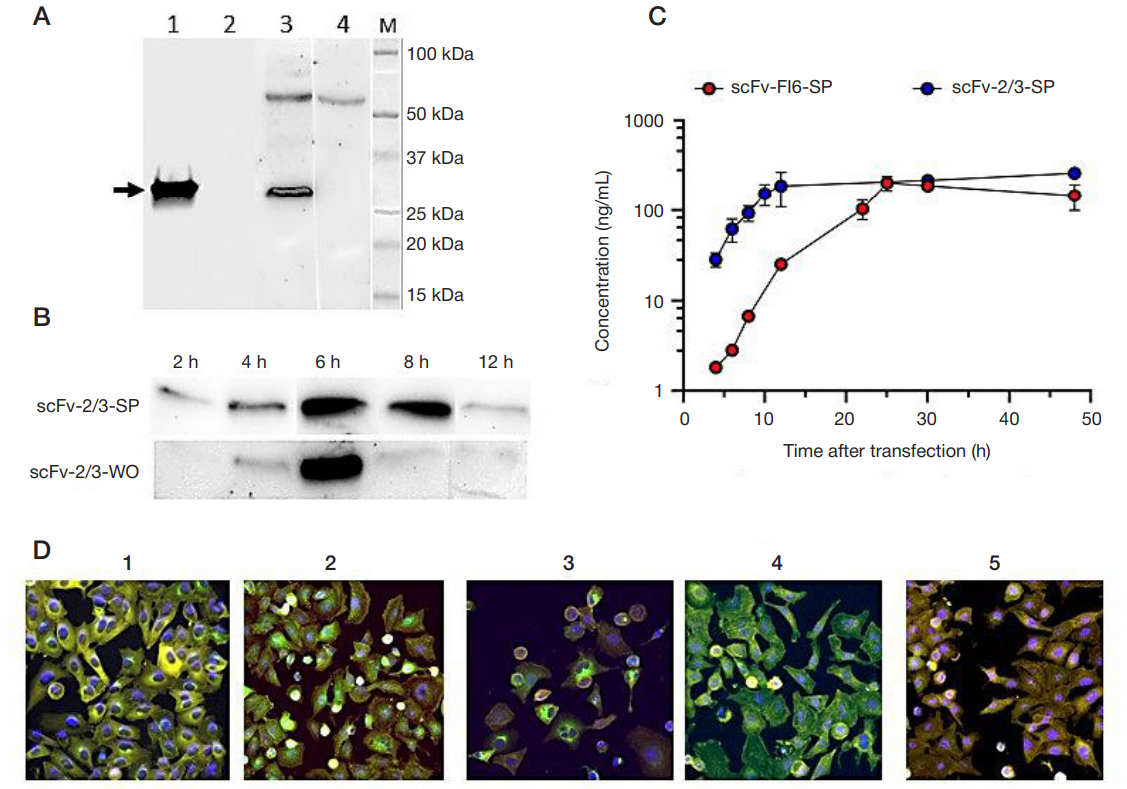
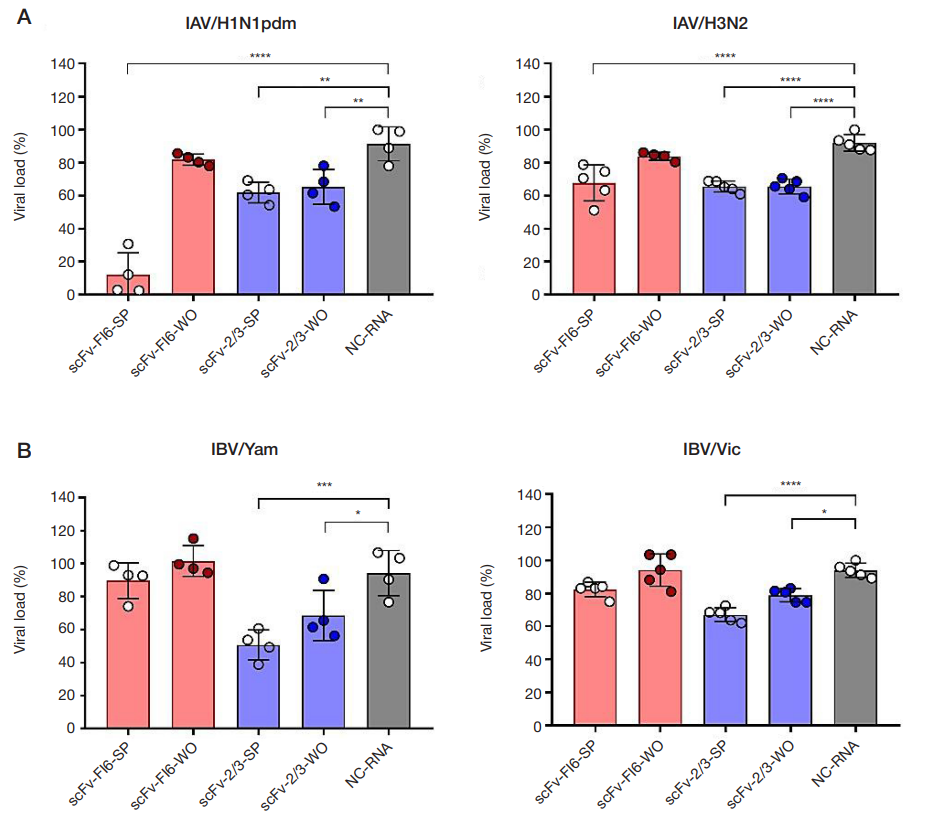
Antiviral activity of mRNAS encoding intracellular scFv antibodies against conserved influenza virus epitopes
Smorodintsev Research Institute of Influenza, St. Petersburg, Russia
Correspondence should be addressed: Sergey A. Klotchenko
Professora Popova, 15/17, Saint Petersburg, 197022, Russia; ur.liam@kitafsof
Funding: This study was supported by financially by the Russian Science Foundation, Agreement No. 24-25-00488: "Study of the antiviral potential of intracellular scFv antibodies against influenza virus" (supervisor — S.A. Klotchenko), https://rscf.ru/project/24-25-00488/
Author contribution: Plotnikova MA — design of structures, conducting experiments, registration and analysis of results, statistical processing, manuscript authoring and formatting; Oleynik VA — conducting experiments, registration of results; Klotchenko SA — study design, preparation and characterization of mRNA preparations, conducting experiments, registration and analysis of results, manuscript editing.