This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (CC BY).
ORIGINAL RESEARCH
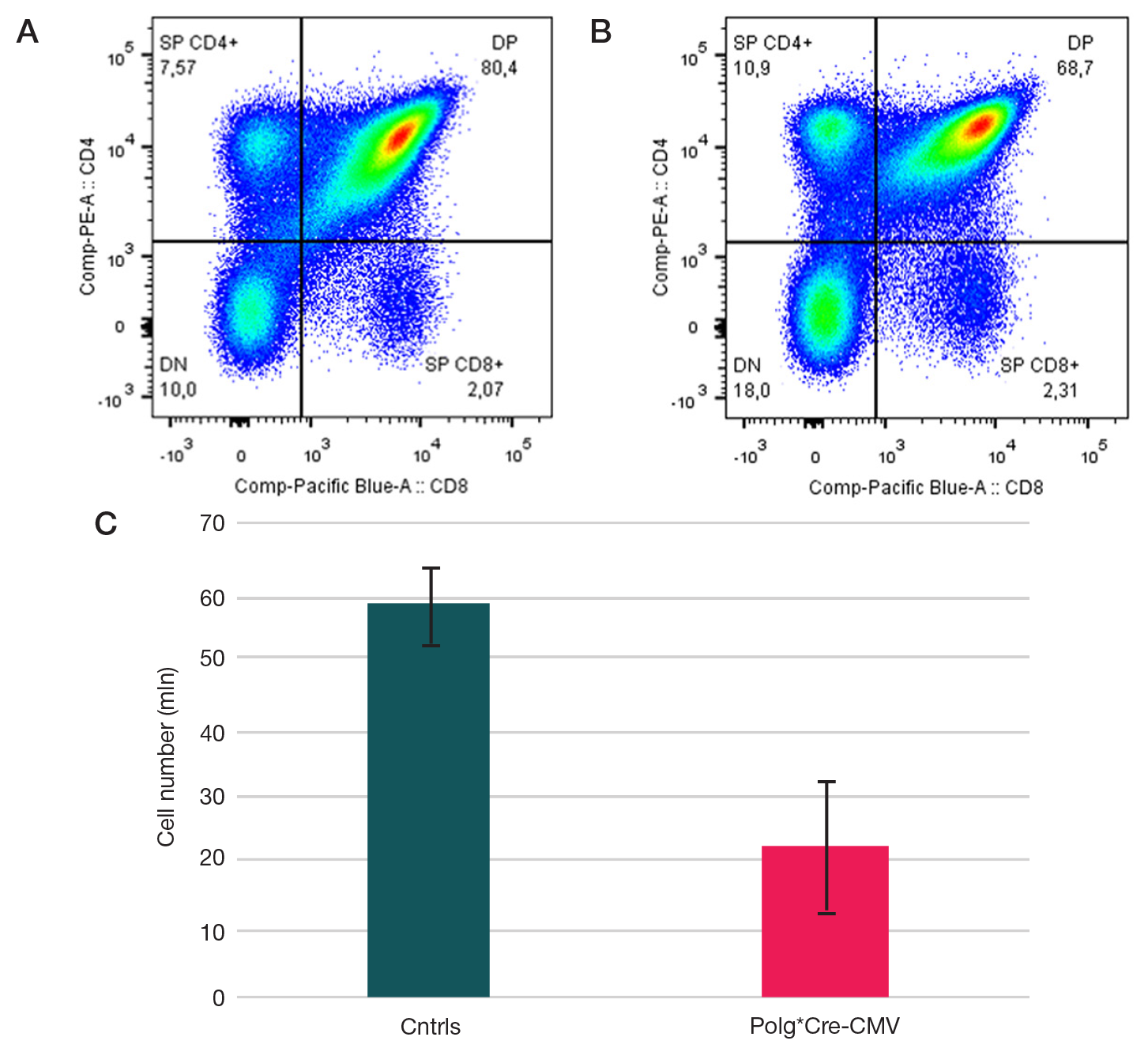
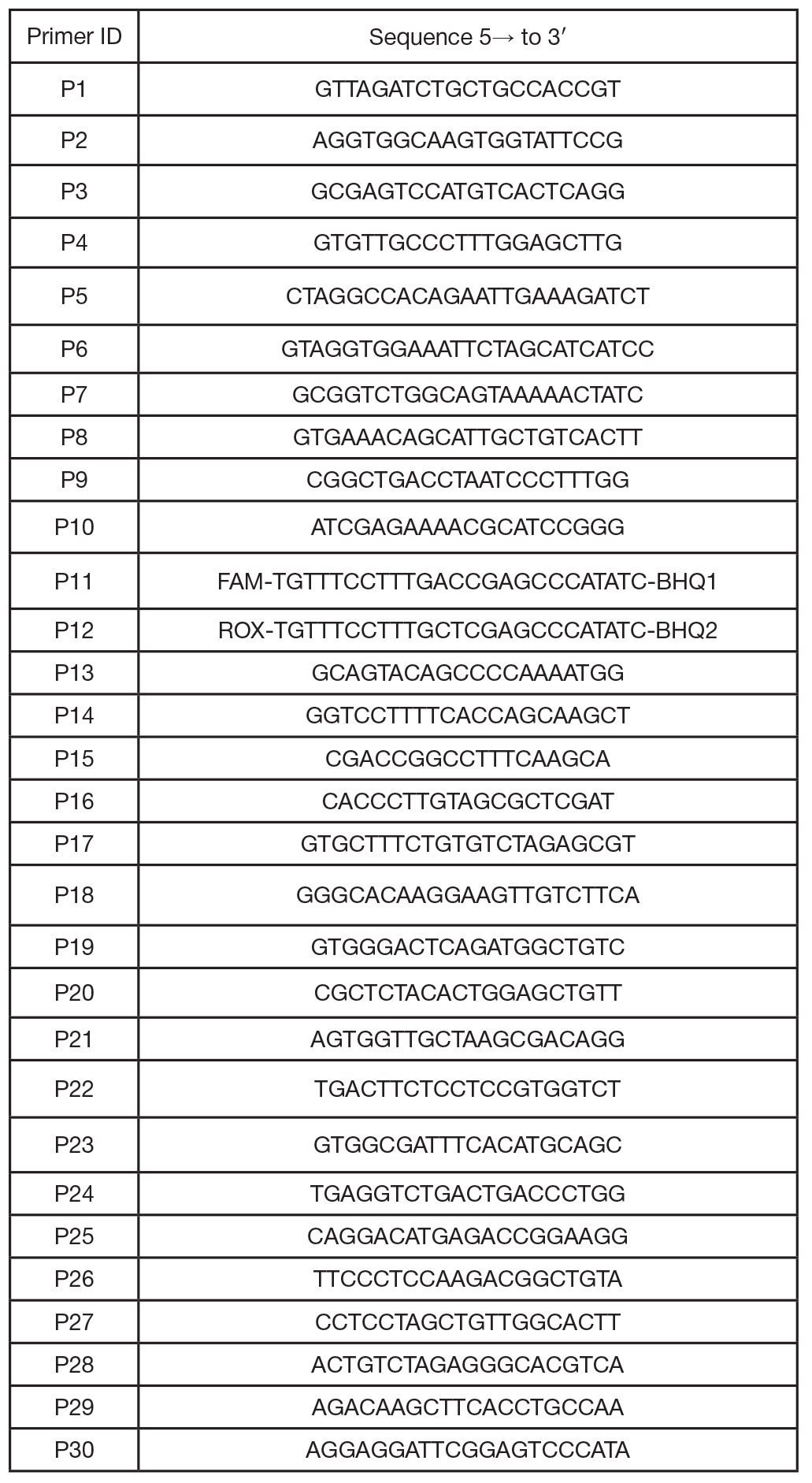
Models of mitochondrial dysfunction with inducible expression of Polg pathogenic mutant variant
1 Institute of Gene Biology, Moscow, Russia
2 Blokhin Russian Cancer Research Center, Moscow, Russia
Correspondence should be addressed: Marina V. Kubekina
Beskudnikovsky bulvar, 32, korpus 1, Moscow, 127474, Russia; moc.liamg@ymukyram
Funding: the study was supported by Russian Foundation for Basic Research, RFBR Project № 19-34-90073.
Author contribution: Kubekina MV — literature analysis, experimental research, data analysis and interpretation, oligo design, manuscript writing; Kalinina AA, Korshunova DS — experimental research; Bruter AV — literature analysis, research planning, data analysis and interpretation; Silaeva YY — literature analysis, research planning, data analysis and interpretation, scientific editing of the manuscript.
Compliance with ethical standards: the study was approved by Ethical Review board at the Institute of Gene Biology (Protocol of 05 December 2021) and carried out in strict compliance with the Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes.