This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (CC BY).
ORIGINAL RESEARCH
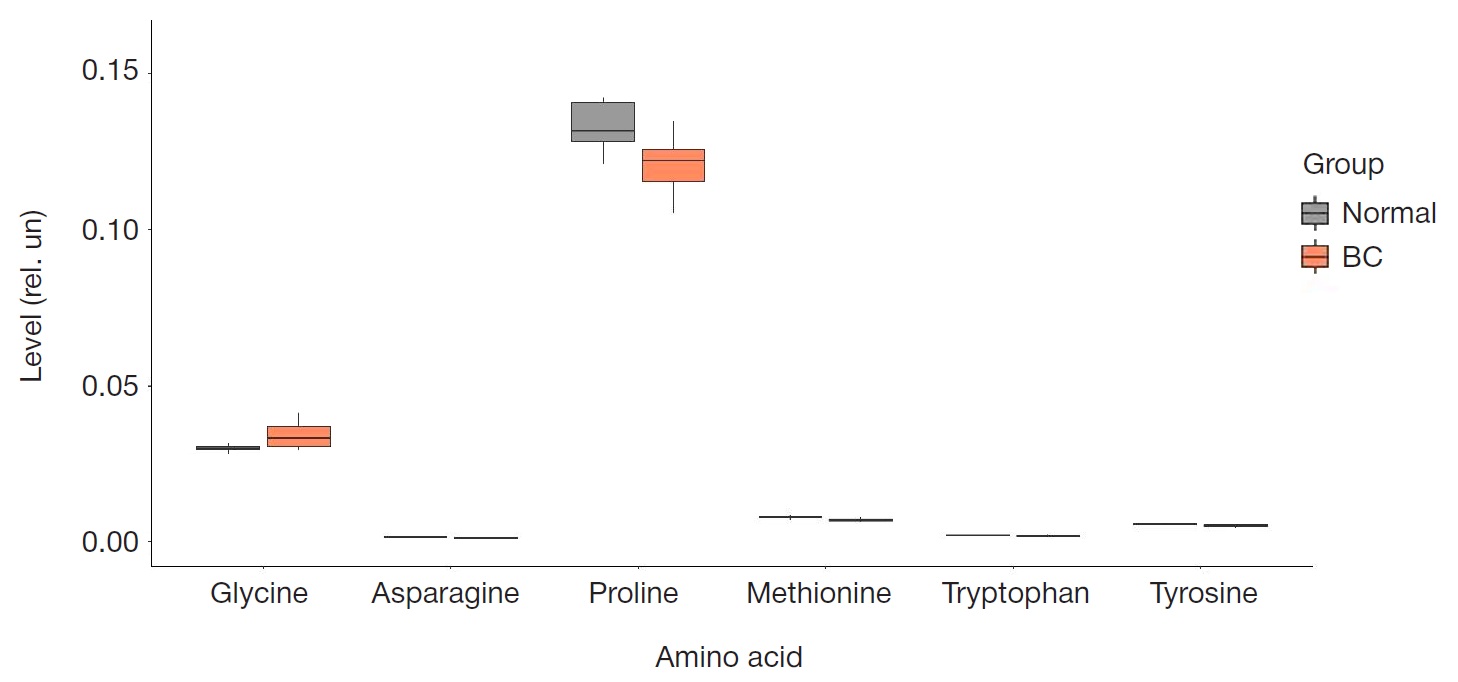
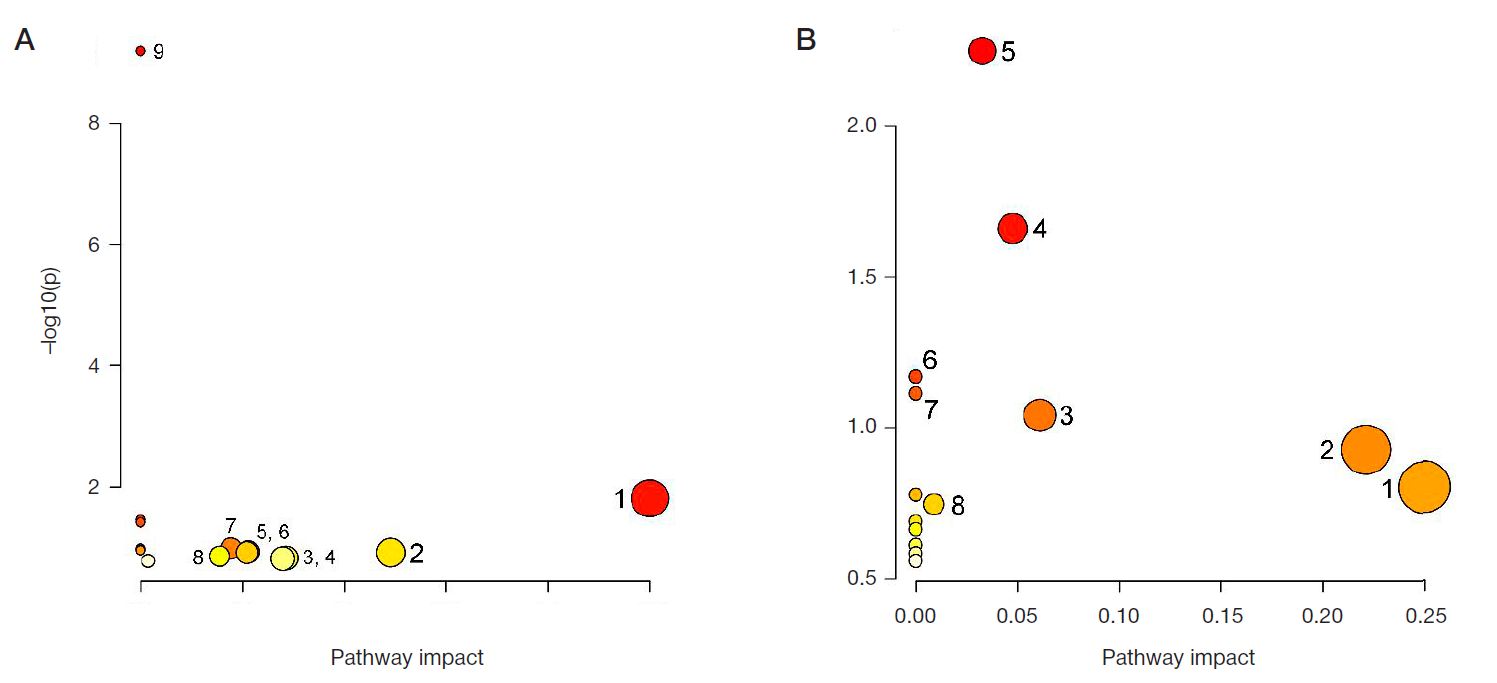
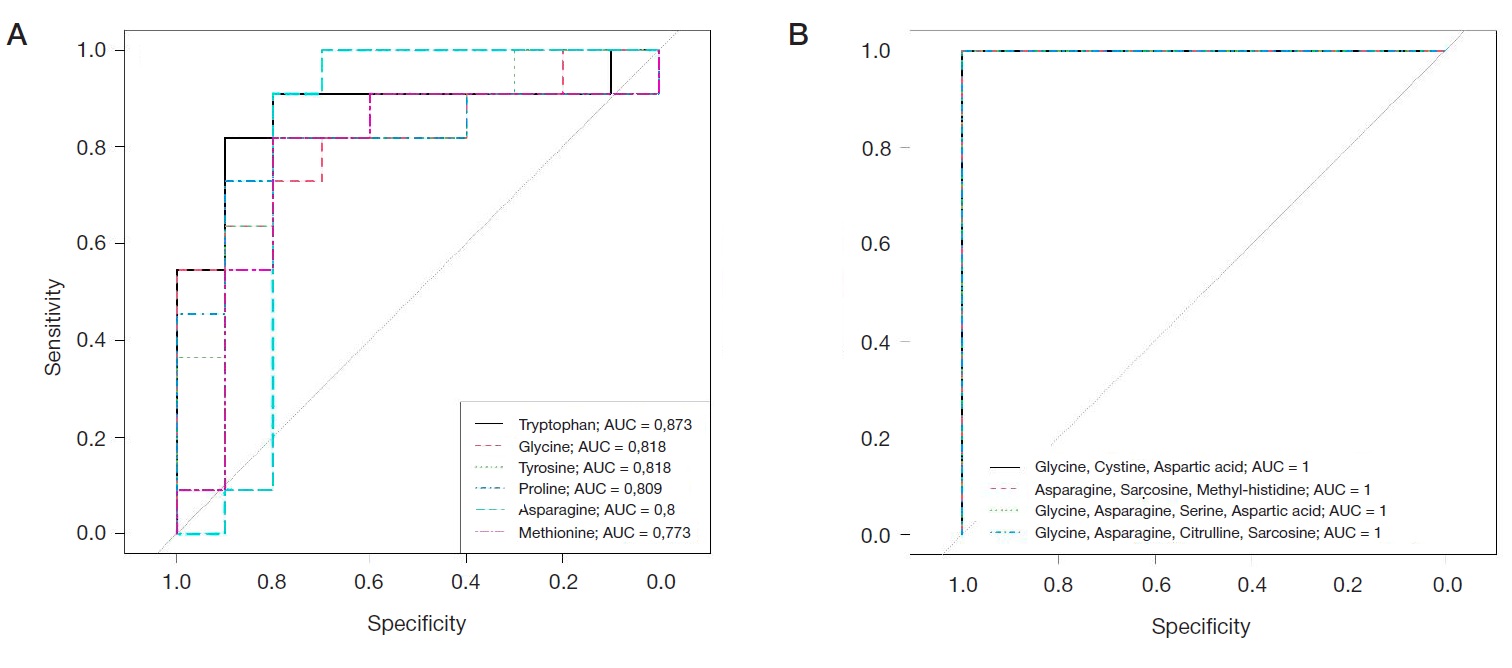
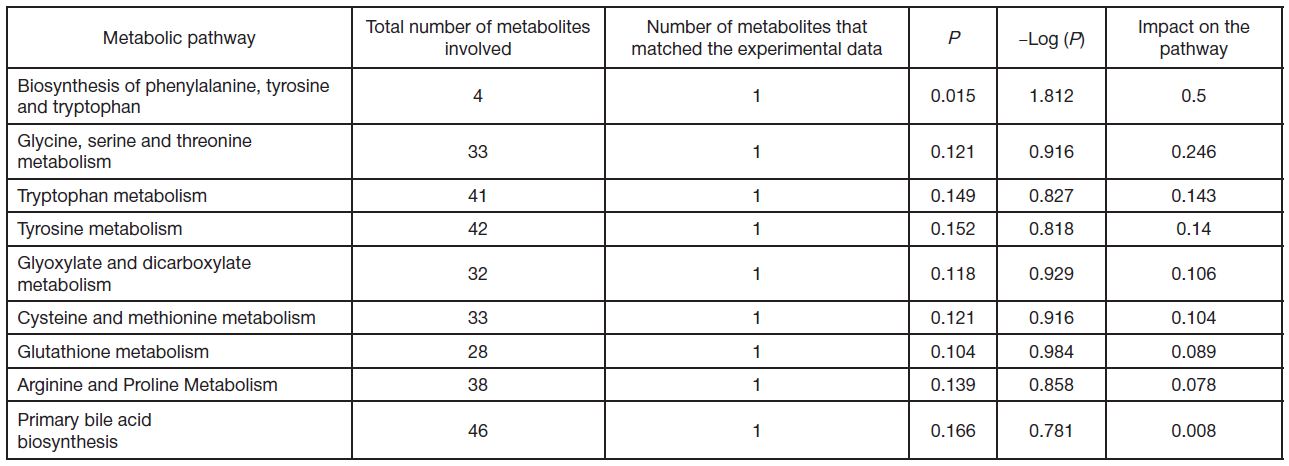
Peculiarities of amino acid profile in monocytes in breast cancer
1 Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Moscow, Russia
2 Cancer Research Institute, Tomsk National Research Medical Center, Tomsk, Russia
3 Tomsk National State University, Tomsk, Russia
4 Siberian State Medical University, Tomsk, Russia
5 Institute of Transfusion Medicine and Immunology, Faculty of Medicine Mannheim, University of Heidelberg, Heidelberg, Germany
6 German Red Cross Blood Service Baden-Württemberg-Hesse, Mannheim, Germany
Correspondence should be addressed: Anastasia V. Novoselova
Academik Oparin street, 4, Moscow, 117198, Russia; moc.liamg@hciveknarfv
Funding: The study was financially supported by the Russian Federation represented by the Ministry of Science and Higher Education of the Russian Federation (agreement dated 29 September 2021 № 075-15-2021-1073 on the topic "Genetic and epigenetic editing of tumor cells and the microenvironment in order to block metastasis ").
Author contribution: Novoselova АV — material processing, monocyte amino acid profile analysis, statistical data processing, text editing; Yushina MN — material processing, text writing and editing; Patysheva MR — study concept and design, monocyte isolation; Prostakishina EA — monocyte isolation; Bragina OD, Garbukov EY — patient selection, collection of biological material; Kzhyshkowska JG — study concept and design.
Compliance with ethical standards: the study is approved by the ethics committee of the Research Institute of Oncology under Tomsk National Research Medical Center (record No.10 dated 05 December 2019), was conducted in accordance with the standards of the ethics committee of V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, federal laws of the Russian Federation (Nos. 152, 323, etc.) and the 1964 Declaration of Helsinki with all subsequent additions and amendments regulating scientific research on biomaterials obtained from humans. All participants signed an informed consent to participate in the study.