This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (CC BY).
ORIGINAL RESEARCH
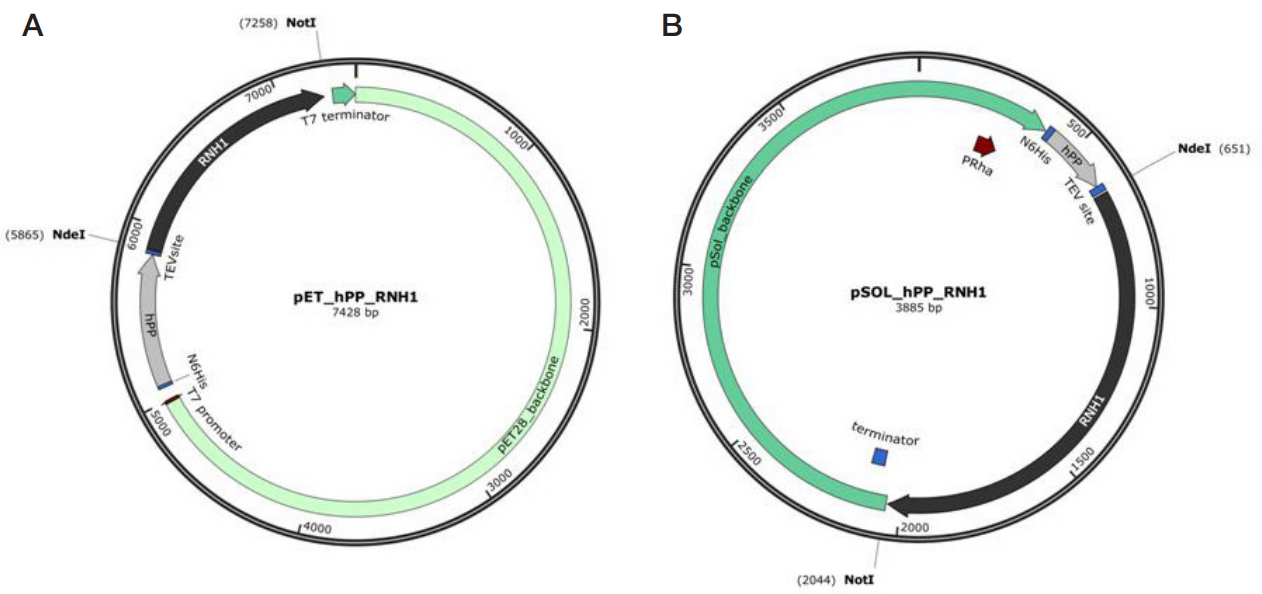
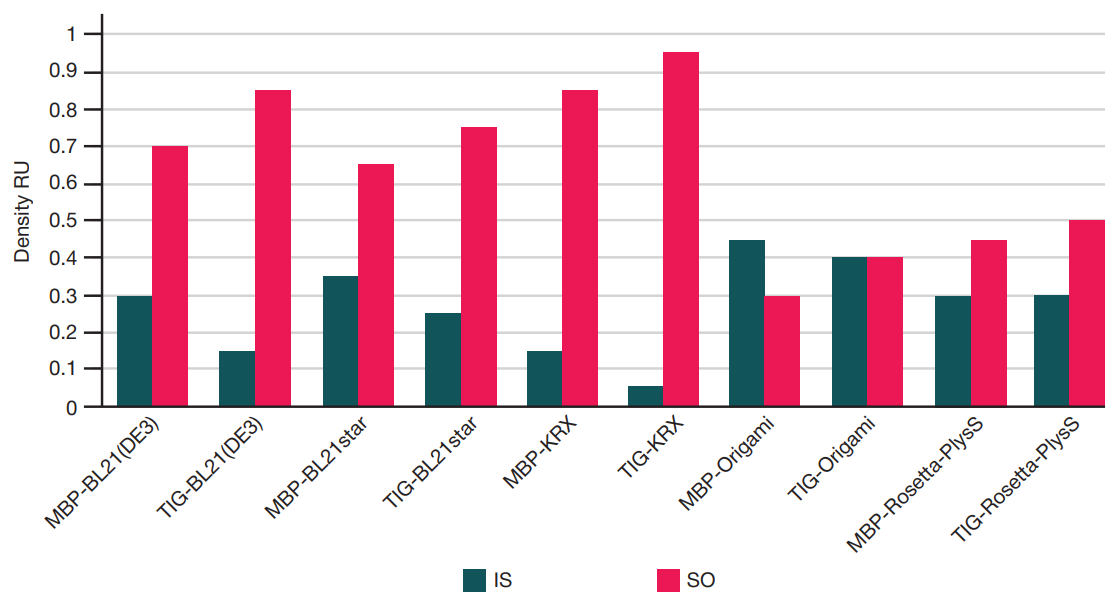
Preparation of a recombinant ribonuclease inhibitor in E. coli for use in mRNA synthesis in vitro
1 Institute of Biochemistry and Physiology of Microorganisms, Russian Academy of Sciences, Pushchino, Russia
2 Sirius University of Science and Technology, Sirius, Sochi, Russia
Correspondence should be addressed: Maxim O. Nagornykh
Prospekt Nauki, 5, Pushchino, 142290, Russia; moc.liamg@rennabred
Funding: the study was financially supported in the context of the program of Ministry of higher education and science of the Russian Federation (agreement #075-10-2021-113, unique project number RF----193021X0001).
Author contribution: Zakharov MV — selection of conditions of production of recombinant proteins, production experiments in different E. coli strains; Zagoskin AA — chromatographic clearing of recombinant proteins; Nagornykh MO — conceptualization, design of genetic constructs, article authoring; Ivanov RA — general management.