This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (CC BY).
ORIGINAL RESEARCH
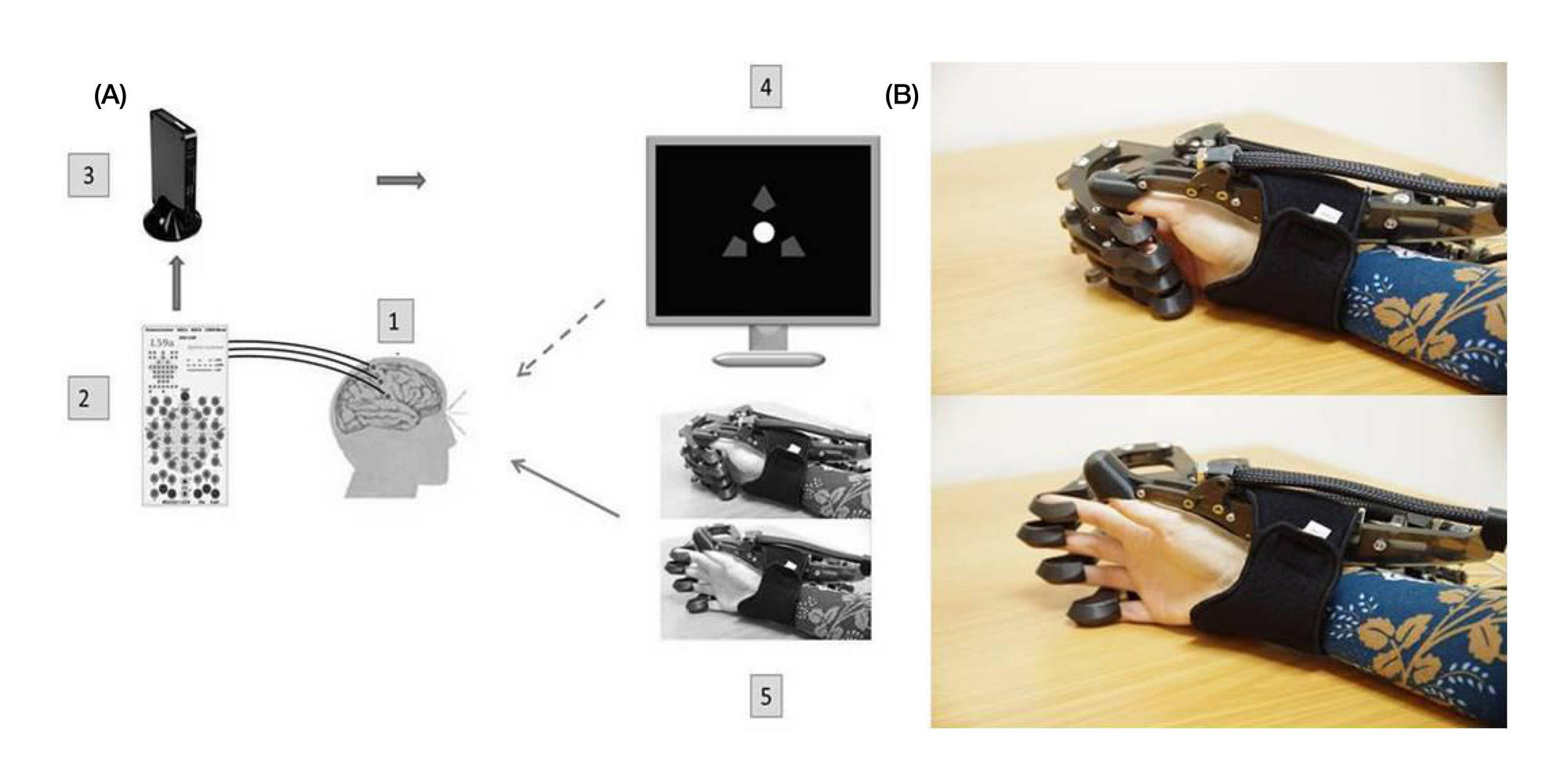
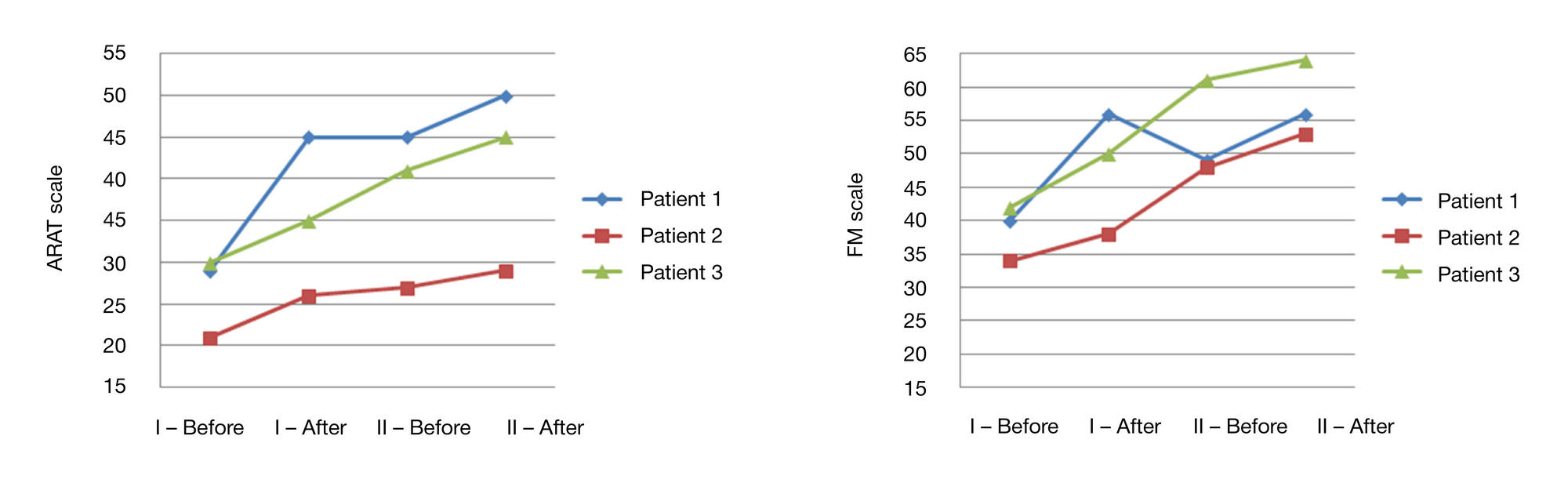
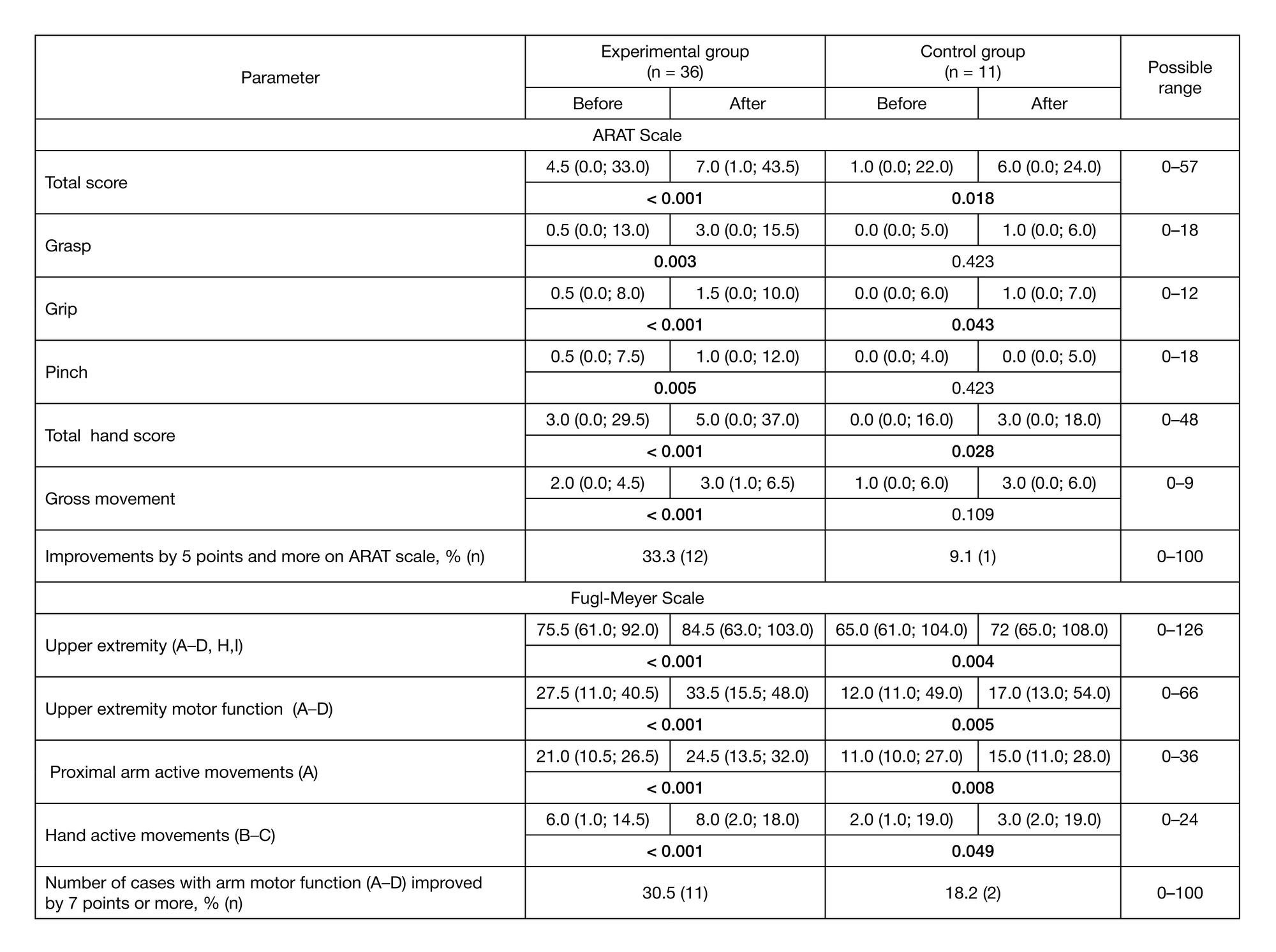
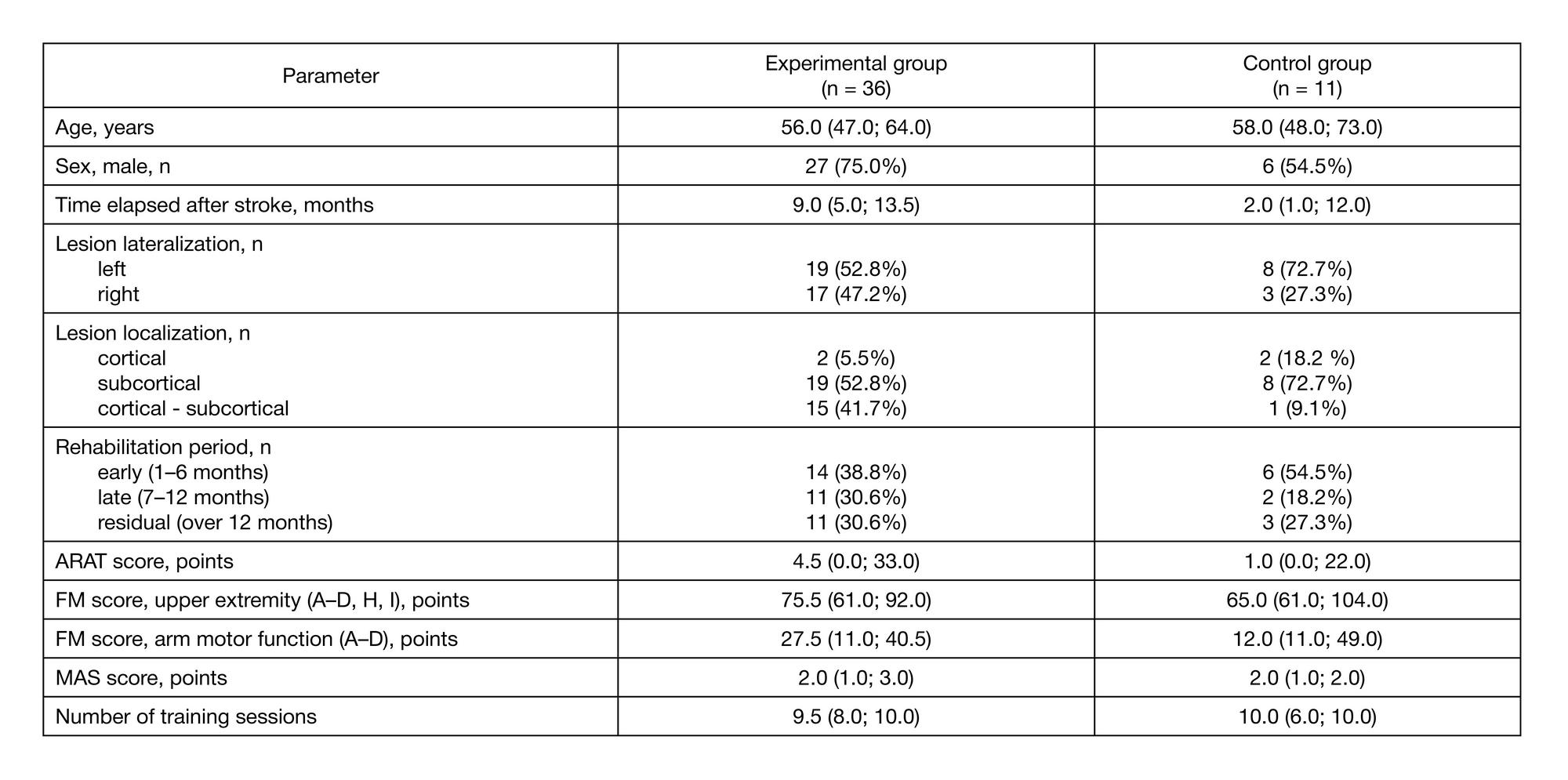
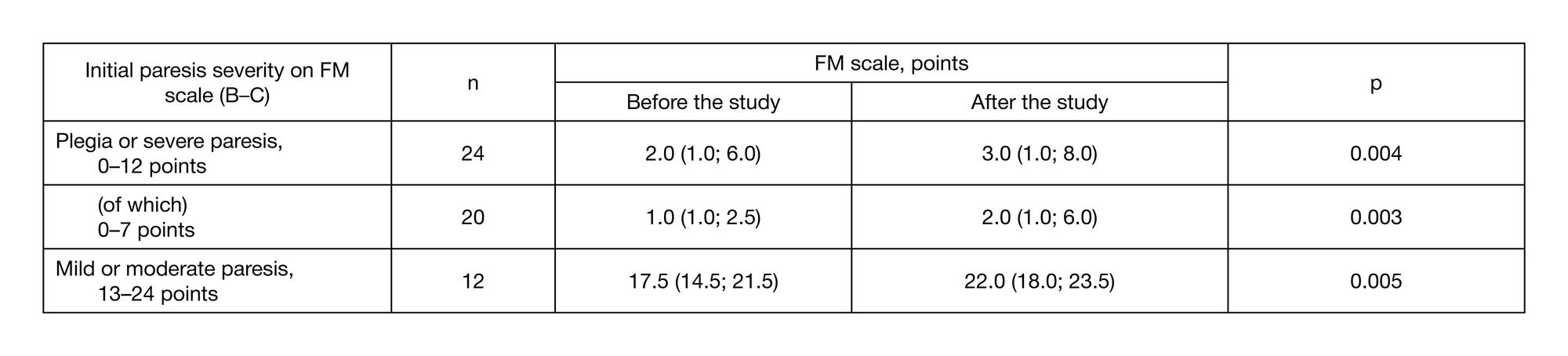
Preliminary results of a controlled study of BCI-exoskeleton technology efficacy in patients with poststroke arm paresis
1 Institute of Higher Nervous Activity and Neurophysiology, RAS, Moscow, Russia
2 Pirogov Russian National Research Medical University, Moscow, Russia
3 Research Center of Neurology, Moscow, Russia
4 Vladimirsky Moscow Regional Research Clinical Institute, Moscow, Russia
5 Municipal Clinical Hospital no. 31, Moscow, Russia
Correspondence should be addressed: Olesya Mokienko
Volokolamskoye shosse, d. 80, kab. 133, Moscow, Russia, 125367; ur.xednay@dm.aysel
Funding: the study was supported by the Ministry of Education and Science of the Russian Federation (Grant Agreement no. 14.607.21.0128 dated October 27, 2015), Russian Foundation Basic Research grants no. 16-04-01506а and 16-04-00962а.